Kaposi's sarcoma herpesvirus K15 protein contributes to virus-induced angiogenesis by recruiting PLCγ1 and activating NFAT1-dependent RCAN1 expression
- PMID: 23028325
- PMCID: PMC3460623
- DOI: 10.1371/journal.ppat.1002927
Kaposi's sarcoma herpesvirus K15 protein contributes to virus-induced angiogenesis by recruiting PLCγ1 and activating NFAT1-dependent RCAN1 expression
Abstract
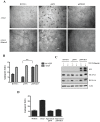
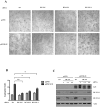
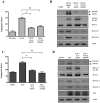
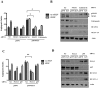
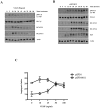
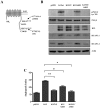
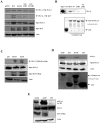
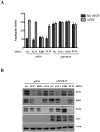
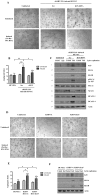
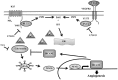
Kaposi's Sarcoma (KS), caused by Kaposi's Sarcoma Herpesvirus (KSHV), is a highly vascularised angiogenic tumor of endothelial cells, characterized by latently KSHV-infected spindle cells and a pronounced inflammatory infiltrate. Several KSHV proteins, including LANA-1 (ORF73), vCyclin (ORF72), vGPCR (ORF74), vIL6 (ORF-K2), vCCL-1 (ORF-K6), vCCL-2 (ORF-K4) and K1 have been shown to exert effects that can lead to the proliferation and atypical differentiation of endothelial cells and/or the secretion of cytokines with angiogenic and inflammatory properties (VEGF, bFGF, IL6, IL8, GROα, and TNFβ). To investigate a role of the KSHV K15 protein in KSHV-mediated angiogenesis, we carried out a genome wide gene expression analysis on primary endothelial cells infected with KSHV wildtype (KSHVwt) and a KSHV K15 deletion mutant (KSHVΔK15). We found RCAN1/DSCR1 (Regulator of Calcineurin 1/Down Syndrome critical region 1), a cellular gene involved in angiogenesis, to be differentially expressed in KSHVwt- vs KSHVΔK15-infected cells. During physiological angiogenesis, expression of RCAN1 in endothelial cells is regulated by VEGF (vascular endothelial growth factor) through a pathway involving the activation of PLCγ1, Calcineurin and NFAT1. We found that K15 directly recruits PLCγ1, and thereby activates Calcineurin/NFAT1-dependent RCAN1 expression which results in the formation of angiogenic tubes. Primary endothelial cells infected with KSHVwt form angiogenic tubes upon activation of the lytic replication cycle. This effect is abrogated when K15 is deleted (KSHVΔK15) or silenced by an siRNA targeting the K15 expression. Our study establishes K15 as one of the KSHV proteins that contribute to KSHV-induced angiogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Inhibiting the Recruitment of PLCγ1 to Kaposi's Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells.PLoS Pathog. 2015 Aug 21;11(8):e1005105. doi: 10.1371/journal.ppat.1005105. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26295810 Free PMC article.
-
The Kaposi's sarcoma-associated herpesvirus (KSHV) non-structural membrane protein K15 is required for viral lytic replication and may represent a therapeutic target.PLoS Pathog. 2017 Sep 22;13(9):e1006639. doi: 10.1371/journal.ppat.1006639. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28938025 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Nonstructural Membrane Protein pK15 Recruits the Class II Phosphatidylinositol 3-Kinase PI3K-C2α To Activate Productive Viral Replication.J Virol. 2018 Aug 16;92(17):e00544-18. doi: 10.1128/JVI.00544-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29950425 Free PMC article.
-
Human Gammaherpesvirus 8 Oncogenes Associated with Kaposi's Sarcoma.Int J Mol Sci. 2022 Jun 29;23(13):7203. doi: 10.3390/ijms23137203. Int J Mol Sci. 2022. PMID: 35806208 Free PMC article. Review.
-
KSHV non-structural membrane proteins involved in the activation of intracellular signaling pathways and the pathogenesis of Kaposi's sarcoma.Curr Opin Virol. 2016 Oct;20:11-19. doi: 10.1016/j.coviro.2016.07.008. Epub 2016 Aug 9. Curr Opin Virol. 2016. PMID: 27518127 Review.
Cited by
-
Inhibiting the Recruitment of PLCγ1 to Kaposi's Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells.PLoS Pathog. 2015 Aug 21;11(8):e1005105. doi: 10.1371/journal.ppat.1005105. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26295810 Free PMC article.
-
An endothelial cell line infected by Kaposi's sarcoma-associated herpes virus (KSHV) allows the investigation of Kaposi's sarcoma and the validation of novel viral inhibitors in vitro and in vivo.J Mol Med (Berl). 2019 Mar;97(3):311-324. doi: 10.1007/s00109-018-01733-1. Epub 2019 Jan 4. J Mol Med (Berl). 2019. PMID: 30610257
-
Quantitative RNAseq analysis of Ugandan KS tumors reveals KSHV gene expression dominated by transcription from the LTd downstream latency promoter.PLoS Pathog. 2018 Dec 17;14(12):e1007441. doi: 10.1371/journal.ppat.1007441. eCollection 2018 Dec. PLoS Pathog. 2018. PMID: 30557332 Free PMC article.
-
Whole-genome sequencing of Kaposi sarcoma-associated herpesvirus (KSHV/HHV8) reveals evidence for two African lineages.Virology. 2022 Mar;568:101-114. doi: 10.1016/j.virol.2022.01.005. Epub 2022 Feb 2. Virology. 2022. PMID: 35152042 Free PMC article.
-
Kaposi Sarcoma, a Trifecta of Pathogenic Mechanisms.Diagnostics (Basel). 2022 May 16;12(5):1242. doi: 10.3390/diagnostics12051242. Diagnostics (Basel). 2022. PMID: 35626397 Free PMC article. Review.
References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266: 1865–1869. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM (1995) Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 332: 1186–1191. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, et al. (1995) Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86: 1276–1280. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

