Transforming growth factor β suppresses osteoblast differentiation via the vimentin activating transcription factor 4 (ATF4) axis
- PMID: 22952236
- PMCID: PMC3476265
- DOI: 10.1074/jbc.M112.372458
Transforming growth factor β suppresses osteoblast differentiation via the vimentin activating transcription factor 4 (ATF4) axis
Abstract
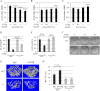
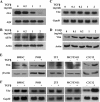
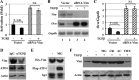
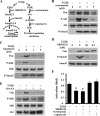
ATF4 is an osteoblast-enriched transcription factor of the leucine zipper family. We recently identified that vimentin, a leucine zipper-containing intermediate filament protein, suppresses ATF4-dependent osteocalcin (Ocn) transcription and osteoblast differentiation. Here we show that TGFβ inhibits ATF4-dependent activation of Ocn by up-regulation of vimentin expression. Osteoblasts lacking Atf4 (Atf4(-/-)) were less sensitive than wild-type (WT) cells to the inhibition by TGFβ on alkaline phosphatase activity, Ocn transcription and mineralization. Importantly, the anabolic effect of a monoclonal antibody neutralizing active TGFβ ligands on bone in WT mice was blunted in Atf4(-/-) mice. These data establish that ATF4 is required for TGFβ-related suppression of Ocn transcription and osteoblast differentiation in vitro and in vivo. Interestingly, TGFβ did not directly regulate the expression of ATF4; instead, it enhanced the expression of vimentin, a negative regulator of ATF4, at the post-transcriptional level. Accordingly, knockdown of endogenous vimentin in 2T3 osteoblasts abolished the inhibition of Ocn transcription by TGFβ, confirming an indirect mechanism by which TGFβ acts through vimentin to suppress ATF4-dependent Ocn activation. Furthermore, inhibition of PI3K/Akt/mTOR signaling, but not canonical Smad signaling, downstream of TGFβ, blocked TGFβ-induced synthesis of vimentin, and inhibited ATF4-dependent Ocn transcription in osteoblasts. Thus, our study identifies that TGFβ stimulates vimentin production via PI3K-Akt-mTOR signaling, which leads to suppression of ATF4-dependent Ocn transcription and osteoblast differentiation.
Figures
Similar articles
-
General transcription factor IIA-gamma increases osteoblast-specific osteocalcin gene expression via activating transcription factor 4 and runt-related transcription factor 2.J Biol Chem. 2008 Feb 29;283(9):5542-53. doi: 10.1074/jbc.M705653200. Epub 2008 Jan 2. J Biol Chem. 2008. PMID: 18171674 Free PMC article.
-
Vimentin inhibits ATF4-mediated osteocalcin transcription and osteoblast differentiation.J Biol Chem. 2009 Oct 30;284(44):30518-25. doi: 10.1074/jbc.M109.052373. Epub 2009 Sep 2. J Biol Chem. 2009. PMID: 19726676 Free PMC article.
-
Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2.J Biol Chem. 2011 Feb 11;286(6):4809-18. doi: 10.1074/jbc.M110.152900. Epub 2010 Dec 6. J Biol Chem. 2011. PMID: 21135100 Free PMC article.
-
Advances in the roles of ATF4 in osteoporosis.Biomed Pharmacother. 2023 Dec 31;169:115864. doi: 10.1016/j.biopha.2023.115864. Epub 2023 Nov 9. Biomed Pharmacother. 2023. PMID: 37948991 Review.
-
FIAT control of osteoblast activity.J Cell Biochem. 2010 Feb 15;109(3):453-9. doi: 10.1002/jcb.22435. J Cell Biochem. 2010. PMID: 20013796 Review.
Cited by
-
TGF-β prevents phosphate-induced osteogenesis through inhibition of BMP and Wnt/β-catenin pathways.PLoS One. 2014 Feb 27;9(2):e89179. doi: 10.1371/journal.pone.0089179. eCollection 2014. PLoS One. 2014. PMID: 24586576 Free PMC article.
-
Activation of mTOR ameliorates fragile X premutation rCGG repeat-mediated neurodegeneration.PLoS One. 2013 Apr 23;8(4):e62572. doi: 10.1371/journal.pone.0062572. Print 2013. PLoS One. 2013. PMID: 23626835 Free PMC article.
-
Mutation in osteoactivin decreases bone formation in vivo and osteoblast differentiation in vitro.Am J Pathol. 2014 Mar;184(3):697-713. doi: 10.1016/j.ajpath.2013.11.031. Epub 2014 Jan 23. Am J Pathol. 2014. PMID: 24462663 Free PMC article.
-
Down-regulated expression of vimentin induced by mechanical stress in fibroblasts derived from patients with ossification of the posterior longitudinal ligament.Eur Spine J. 2014 Nov;23(11):2410-5. doi: 10.1007/s00586-014-3394-8. Epub 2014 Jun 8. Eur Spine J. 2014. PMID: 24908253
-
A Loop-Based and AGO-Incorporated Virtual Screening Model Targeting AGO-Mediated miRNA-mRNA Interactions for Drug Discovery to Rescue Bone Phenotype in Genetically Modified Mice.Adv Sci (Weinh). 2020 May 28;7(13):1903451. doi: 10.1002/advs.201903451. eCollection 2020 Jul. Adv Sci (Weinh). 2020. PMID: 32670749 Free PMC article.
References
-
- Lucas P. A. (1989) Chemotactic response of osteoblast-like cells to transforming growth factor β. Bone 10, 459–463 - PubMed
-
- Pfeilschifter J., Wolf O., Naumann A., Minne H. W., Mundy G. R., Ziegler R. (1990) Chemotactic response of osteoblastlike cells to transforming growth factor β. J. Bone Miner. Res. 5, 825–830 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

