4α-phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4
- PMID: 22928864
- PMCID: PMC3579293
- DOI: 10.1111/j.1476-5381.2012.02186.x
4α-phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4
Abstract
Background and purpose: The Ca(2+) -permeable cation channel TRPV4 is activated by mechanical disturbance of the cell membrane and is implicated in mechanical hyperalgesia. Nerve growth factor (NGF) is increased during inflammation and causes mechanical hyperalgesia. 4α-phorbol 12,13-didecanoate (4αPDD) has been described as a selective TRPV4 agonist. We investigated NGF-induced hyperalgesia in TRPV4 wild-type (+/+) and knockout (-/-) mice, and the increases in [Ca(2+) ](i) produced by 4αPDD in cultured mouse dorsal root ganglia neurons following exposure to NGF.
Experimental approach: Withdrawal thresholds to heat, von Frey hairs and pressure were measured in mice before and after systemic administration of NGF. Changes in intracellular Ca(2+) concentration were measured by ratiometric imaging with Fura-2 in cultured DRG and trigeminal ganglia (TG) neurons during perfusion of TRPV4 agonists.
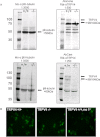
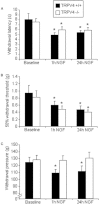
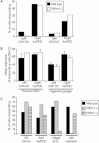
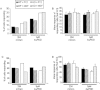
Key results: Administration of NGF caused a significant sensitization to heat and von Frey stimuli in TRPV4 +/+ and -/- mice, but only TRPV4 +/+ mice showed sensitization to noxious pressure. 4αPDD stimulated a dose-dependent increase in [Ca(2+) ](i) in neurons from +/+ and -/- mice, with the proportion of responding neurons and magnitude of increase unaffected by the genotype. In contrast, the selective TRPV4 agonist GSK1016790A failed to stimulate an increase in intracellular Ca(2+) in cultured neurons. Responses to 4αPDD were unaffected by pretreatment with NGF.
Conclusions and implications: TRPV4 contributes to mechanosensation in vivo, but there is little evidence for functional TRPV4 in cultured DRG and TG neurons. We conclude that 4αPDD activates these neurons independently of TRPV4, so it is not appropriate to refer to 4αPDD as a selective TRPV4 agonist.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures



Similar articles
-
Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice.J Physiol. 2007 Feb 1;578(Pt 3):715-33. doi: 10.1113/jphysiol.2006.121111. Epub 2006 Nov 23. J Physiol. 2007. PMID: 17124270 Free PMC article.
-
Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms.Gastroenterology. 2008 Sep;135(3):937-46, 946.e1-2. doi: 10.1053/j.gastro.2008.05.024. Epub 2008 May 10. Gastroenterology. 2008. PMID: 18565335
-
TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization.J Neurosci. 2009 May 13;29(19):6217-28. doi: 10.1523/JNEUROSCI.0893-09.2009. J Neurosci. 2009. PMID: 19439599 Free PMC article.
-
Neurotrophic Factors and Nociceptor Sensitization.In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. PMID: 21882462 Free Books & Documents. Review.
-
Transient Receptor Potential Vanilloid (TRPV4) channel inhibition: A novel promising approach for the treatment of lung diseases.Biomed Pharmacother. 2023 Jul;163:114861. doi: 10.1016/j.biopha.2023.114861. Epub 2023 May 11. Biomed Pharmacother. 2023. PMID: 37178575 Review.
Cited by
-
TRPV4 receptor as a functional sensory molecule in bladder urothelium: Stretch-independent, tissue-specific actions and pathological implications.FASEB J. 2020 Jan;34(1):263-286. doi: 10.1096/fj.201900961RR. Epub 2019 Nov 21. FASEB J. 2020. PMID: 31914645 Free PMC article.
-
Role of Known Transient Receptor Potential Vanilloid Channels in Modulating Cardiac Mechanobiology.Front Physiol. 2021 Oct 15;12:734113. doi: 10.3389/fphys.2021.734113. eCollection 2021. Front Physiol. 2021. PMID: 34867442 Free PMC article. Review.
-
TRPing to the Point of Clarity: Understanding the Function of the Complex TRPV4 Ion Channel.Cells. 2021 Jan 15;10(1):165. doi: 10.3390/cells10010165. Cells. 2021. PMID: 33467654 Free PMC article. Review.
-
Peripheral transient receptor potential vanilloid type 4 hypersensitivity contributes to chronic sickle cell disease pain.Pain. 2023 Aug 1;164(8):1874-1886. doi: 10.1097/j.pain.0000000000002889. Epub 2023 Mar 10. Pain. 2023. PMID: 36897169 Free PMC article.
-
Transient receptor potential vanilloid 4 channels as therapeutic targets in diabetes and diabetes-related complications.J Diabetes Investig. 2020 Jul;11(4):757-769. doi: 10.1111/jdi.13244. Epub 2020 Apr 16. J Diabetes Investig. 2020. PMID: 32129549 Free PMC article. Review.
References
-
- Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. 946.e1–e2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

