A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection
- PMID: 22918156
- PMCID: PMC3480968
- DOI: 10.1097/QAI.0b013e31826e7d0f
A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection
Abstract
Objectives: To determine whether intensification with raltegravir improves endothelial function in antiretroviral-treated HIV-infected individuals.
Design: : Randomized, double-blinded, placebo-controlled study.
Methods: Fifty-six subjects with treatment-mediated viral suppression for at least 1 year were randomized to add 400 mg of raltegravir twice daily or matching placebo for 24 weeks. The primary endpoint was the difference in rate of change in endothelial function [as assessed by flow-mediated vasodilation (FMD) of the brachial artery] from baseline to week 24 between the raltegravir and placebo groups. Linear mixed models were used to evaluate the association of treatment group with changes in FMD, immune activation, and measures of viral persistence.
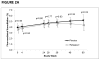
Results: At baseline, the median CD4 T-cell count was 498 cells/mm, nadir CD4 T-cell count was 191 cells/mm, duration of HIV infection was 18 years, FMD was 3.3%, and hyperemic velocity (a marker of microvascular function) was 68.3 cm. There were no significant differences between treatment groups in rate of change in FMD (raltegravir group: +0.032% per week, placebo group: +0.023% per week; P = 0.60). There were also no differences between treatment groups in rate of change in hyperemic velocity, immune activation, or viral persistence. In multivariable analysis, older age, longer duration of HIV infection, and current abacavir use were associated with lower FMD. Lower CD4 T-cell count and current abacavir use were associated with lower hyperemic velocity.
Conclusions: The addition of raltegravir to suppressive antiretroviral therapy did not have a significant impact on cardiovascular risk, as assessed by endothelial function (ClinicalTrials.gov NCT00843713).
Conflict of interest statement
RS, YW, KH, KM, RH, ES, SP, JNM, MPB, PYH: No conflicts.
Figures


Similar articles
-
The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial.PLoS Med. 2010 Aug 10;7(8):e1000321. doi: 10.1371/journal.pmed.1000321. PLoS Med. 2010. PMID: 20711481 Free PMC article. Clinical Trial.
-
Intensification of antiretroviral therapy with raltegravir or addition of hyperimmune bovine colostrum in HIV-infected patients with suboptimal CD4+ T-cell response: a randomized controlled trial.J Infect Dis. 2011 Nov 15;204(10):1532-40. doi: 10.1093/infdis/jir559. Epub 2011 Sep 19. J Infect Dis. 2011. PMID: 21930607 Clinical Trial.
-
Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study.Antivir Ther. 2012;17(2):355-64. doi: 10.3851/IMP1917. Epub 2011 Sep 28. Antivir Ther. 2012. PMID: 22290239 Clinical Trial.
-
Raltegravir: in treatment-naive patients with HIV-1 infection.Drugs. 2010 Mar 26;70(5):631-42. doi: 10.2165/11204590-000000000-00000. Drugs. 2010. PMID: 20329808 Review.
-
Raltegravir: a review of its use in the management of HIV-1 infection in children and adolescents.Paediatr Drugs. 2014 Feb;16(1):91-100. doi: 10.1007/s40272-013-0058-9. Paediatr Drugs. 2014. PMID: 24277175 Review.
Cited by
-
The role of HIV integration in viral persistence: no more whistling past the proviral graveyard.J Clin Invest. 2016 Feb;126(2):438-47. doi: 10.1172/JCI80564. Epub 2016 Feb 1. J Clin Invest. 2016. PMID: 26829624 Free PMC article. Review.
-
HIV infection, antiretroviral therapy, and measures of endothelial function, inflammation, metabolism, and oxidative stress.PLoS One. 2017 Aug 17;12(8):e0183511. doi: 10.1371/journal.pone.0183511. eCollection 2017. PLoS One. 2017. PMID: 28817706 Free PMC article.
-
Immune activation and HIV persistence: considerations for novel therapeutic interventions.Curr Opin HIV AIDS. 2013 May;8(3):211-6. doi: 10.1097/COH.0b013e32835f9788. Curr Opin HIV AIDS. 2013. PMID: 23454864 Free PMC article. Review.
-
T-cell exhaustion in HIV infection.Immunol Rev. 2019 Nov;292(1):149-163. doi: 10.1111/imr.12823. Immunol Rev. 2019. PMID: 31883174 Free PMC article. Review.
-
Why the HIV Reservoir Never Runs Dry: Clonal Expansion and the Characteristics of HIV-Infected Cells Challenge Strategies to Cure and Control HIV Infection.Viruses. 2021 Dec 14;13(12):2512. doi: 10.3390/v13122512. Viruses. 2021. PMID: 34960781 Free PMC article. Review.
References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. - PubMed
-
- Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004 Apr 6;109(13):1603–1608. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 HL095130/HL/NHLBI NIH HHS/United States
- P30 MH062246/MH/NIMH NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- R21 AI055273/AI/NIAID NIH HHS/United States
- K23AI075985/AI/NIAID NIH HHS/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- R01 AI087145/AI/NIAID NIH HHS/United States
- AI055273/AI/NIAID NIH HHS/United States
- K23 AI075985/AI/NIAID NIH HHS/United States
- P30 MH62246/MH/NIMH NIH HHS/United States
- AI052745/AI/NIAID NIH HHS/United States
- K24AI069994/AI/NIAID NIH HHS/United States
- K24 RR016482/RR/NCRR NIH HHS/United States
- R01 AI052745/AI/NIAID NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- R01 AI057020/AI/NIAID NIH HHS/United States
- RR 16482/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

