Cell-free circulating plasma hTERT mRNA is a useful marker for prostate cancer diagnosis and is associated with poor prognosis tumor characteristics
- PMID: 22916267
- PMCID: PMC3423343
- DOI: 10.1371/journal.pone.0043470
Cell-free circulating plasma hTERT mRNA is a useful marker for prostate cancer diagnosis and is associated with poor prognosis tumor characteristics
Abstract
Background: Serum prostate-specific antigen (PSA) is the most widely used marker for diagnosing prostate cancer (PCa). It lacks specificity and predictive value, resulting in inaccurate diagnoses and overtreatment of the disease. The aim of this study was to assess the usefulness of plasma telomerase reverse transcriptase (hTERT) mRNA as a diagnostic and prognostic tool for PCa and its association with clinicopathological parameters of tumors.
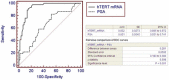
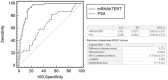
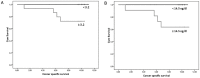
Principal findings: Plasma hTERT mRNA levels were determined by qRT-PCR in 105 consecutive patients with elevated PSA levels and in 68 healthy volunteers. The diagnostic accuracy, the efficacy as a prognostic factor of biochemical recurrence and the association with tumor clinicopathological parameters of plasma hTERT mRNA and serum PSA tests were determined using univariate and multivariate analyses. The results show that plasma hTERT mRNA is a non-invasive biomarker for PCa diagnosis that shows higher sensitivity (85% vs. 83%), specificity (90% vs. 47%), positive predictive value (83% vs. 56%), and negative predictive value (92% vs. 77%) than serum PSA. Plasma hTERT mRNA is significantly associated with poor prognosis tumor clinicopathological parameters and is a significant independent predictor of PCa (p<0.0001). Univariate analysis identified plasma hTERT mRNA (but not serum PSA) as a significant prognostic factor of biochemical recurrence. Plasma hTERT mRNA Kaplan-Meier curves confirmed the significant differences between groups and patients with higher levels than the cut-off value showed diminished recurrence-free survival (p=0.004), whereas no differences were observed with serum PSA (p=0.38). Multivariate analysis indicated that plasma hTERT mRNA (but not serum PSA) and stage were significantly associated with biochemical recurrence.
Conclusions: Overall, these findings indicate that hTERT mRNA is a useful non-invasive tumor marker for the molecular diagnosis of PCa, affording a greater diagnostic and prognostic accuracy than the PSA assay and may be of relevance in the follow-up of the disease.
Conflict of interest statement
Figures

Similar articles
-
Plasma hTERT mRNA discriminates between clinically localized and locally advanced disease and is a predictor of recurrence in prostate cancer patients.Expert Opin Biol Ther. 2012 Jun;12 Suppl 1:S69-77. doi: 10.1517/14712598.2012.685716. Epub 2012 May 5. Expert Opin Biol Ther. 2012. PMID: 22559196
-
Real-time quantification of human telomerase reverse transcriptase mRNA in the plasma of patients with prostate cancer.Ann N Y Acad Sci. 2006 Sep;1075:204-10. doi: 10.1196/annals.1368.028. Ann N Y Acad Sci. 2006. PMID: 17108213
-
Prognostic Significance of Serum PSA Level and Telomerase, VEGF and GLUT-1 Protein Expression for the Biochemical Recurrence in Prostate Cancer Patients after Radical Prostatectomy.Pathol Oncol Res. 2020 Apr;26(2):1049-1056. doi: 10.1007/s12253-019-00659-4. Epub 2019 Apr 15. Pathol Oncol Res. 2020. PMID: 30989489 Free PMC article.
-
Clinical usefulness of serum telomerase reverse transcriptase (hTERT) mRNA and epidermal growth factor receptor (EGFR) mRNA as a novel tumor marker for lung cancer.Cancer Sci. 2006 Dec;97(12):1366-73. doi: 10.1111/j.1349-7006.2006.00342.x. Cancer Sci. 2006. PMID: 17052260 Free PMC article.
-
[Proteome-based diagnostic and prognostic biomarkers of prostate cancer].Urologe A. 2013 Sep;52(9):1251-5. doi: 10.1007/s00120-013-3308-0. Urologe A. 2013. PMID: 24026060 Review. German.
Cited by
-
The Prospect and Challenges to the Flow of Liquid Biopsy in Africa.Cells. 2019 Aug 9;8(8):862. doi: 10.3390/cells8080862. Cells. 2019. PMID: 31404988 Free PMC article. Review.
-
Endosomal gene expression: a new indicator for prostate cancer patient prognosis?Oncotarget. 2015 Nov 10;6(35):37919-29. doi: 10.18632/oncotarget.6114. Oncotarget. 2015. PMID: 26473288 Free PMC article.
-
Liquid Biopsy Biomarkers in Bladder Cancer: A Current Need for Patient Diagnosis and Monitoring.Int J Mol Sci. 2018 Aug 24;19(9):2514. doi: 10.3390/ijms19092514. Int J Mol Sci. 2018. PMID: 30149597 Free PMC article. Review.
-
RNA Biomarkers: Frontier of Precision Medicine for Cancer.Noncoding RNA. 2017 Feb 20;3(1):9. doi: 10.3390/ncrna3010009. Noncoding RNA. 2017. PMID: 29657281 Free PMC article. Review.
-
Targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer.Oncotarget. 2017 Jan 17;8(3):4960-4976. doi: 10.18632/oncotarget.13634. Oncotarget. 2017. PMID: 27903962 Free PMC article.
References
-
- Jemal A, Siegl R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J Clin 60: 277–300. - PubMed
-
- Hoffman RM (2011) Screening for prostate cancer. N Engl J Med 365: 2013–2019. - PubMed
-
- Gomella LG, Liu XS, Trabulsi EJ, Kelly WK, Myers R, et al. (2011) Screening for prostate cancer: the current evidence and guidelines controversy. Can J Urol 18: 5875–5883. - PubMed
-
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, et al. (2004) Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med 350: 2239–2246. - PubMed
-
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, et al. (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360: 1320–1328. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

