Epstein-Barr virus immortalization of human B-cells leads to stabilization of hypoxia-induced factor 1 alpha, congruent with the Warburg effect
- PMID: 22848707
- PMCID: PMC3407085
- DOI: 10.1371/journal.pone.0042072
Epstein-Barr virus immortalization of human B-cells leads to stabilization of hypoxia-induced factor 1 alpha, congruent with the Warburg effect
Abstract
Background: Epstein-Barr virus (EBV) encodes six nuclear transformation-associated proteins that induce extensive changes in cellular gene expression and signaling and induce B-cell transformation. The role of HIF1A in EBV-induced B-cell immortalization has not been previously studied.
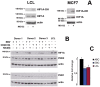
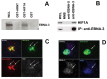
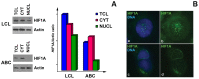
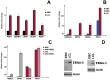
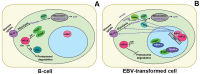
Methods and findings: Using Western blotting and Q-PCR, we found that HIF1A protein is stabilized in EBV-transformed lymphoblastoid cells. Western blotting, GST pulldown assays, and immunoprecipitation showed that EBV-encoded nuclear antigens EBNA-5 and EBNA-3 bind to prolylhydroxylases 1 and 2, respectively, thus inhibiting HIF1A hydroxylation and degradation. Immunostaining and Q-PCR showed that the stabilized HIF1A translocates to the nucleus, forms a heterodimer with ARNT, and transactivates several genes involved in aerobic glycolysis. Using biochemical assays and Q-PCR, we also found that lymphoblastoid cells produce high levels of lactate, lactate dehydrogenase and pyruvate.
Conclusions: Our data suggest that activation of the aerobic glycolytic pathway, corresponding to the Warburg effect, occurs in EBV-transformed lymphoblastoid cells, in contrast to mitogen-activated B-cells.
Conflict of interest statement
Figures

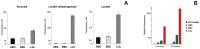
Similar articles
-
Oxidative stress enables Epstein-Barr virus-induced B-cell transformation by posttranscriptional regulation of viral and cellular growth-promoting factors.Oncogene. 2016 Jul 21;35(29):3807-16. doi: 10.1038/onc.2015.450. Epub 2015 Nov 23. Oncogene. 2016. PMID: 26592445
-
EBNA-3B- and EBNA-3C-regulated cellular genes in Epstein-Barr virus-immortalized lymphoblastoid cell lines.J Virol. 2006 Oct;80(20):10139-50. doi: 10.1128/JVI.00854-06. J Virol. 2006. PMID: 17005691 Free PMC article.
-
Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation.J Virol. 1991 May;65(5):2545-54. doi: 10.1128/JVI.65.5.2545-2554.1991. J Virol. 1991. PMID: 1850028 Free PMC article.
-
Immortalizing genes of Epstein-Barr virus.Adv Virus Res. 1991;40:19-55. doi: 10.1016/s0065-3527(08)60276-6. Adv Virus Res. 1991. PMID: 1659776 Review.
-
Nuclear receptors and their role in Epstein -- Barr virus induced B cell transformation.Exp Oncol. 2009 Jun;31(2):67-73. Exp Oncol. 2009. PMID: 19550394 Review.
Cited by
-
Taking over Cellular Energy-Metabolism for TBSV Replication: The High ATP Requirement of an RNA Virus within the Viral Replication Organelle.Viruses. 2020 Jan 3;12(1):56. doi: 10.3390/v12010056. Viruses. 2020. PMID: 31947719 Free PMC article. Review.
-
Hypoxia-inducible factor-1α activation in HPV-positive head and neck squamous cell carcinoma cell lines.Oncotarget. 2017 Sep 11;8(52):89681-89691. doi: 10.18632/oncotarget.20813. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163780 Free PMC article.
-
How lactate affects immune strategies in lymphoma.Front Mol Biosci. 2024 Oct 11;11:1480884. doi: 10.3389/fmolb.2024.1480884. eCollection 2024. Front Mol Biosci. 2024. PMID: 39464313 Free PMC article. Review.
-
Impaired Neonatal Immunity and Infection Resistance Following Fetal Growth Restriction in Preterm Pigs.Front Immunol. 2020 Aug 13;11:1808. doi: 10.3389/fimmu.2020.01808. eCollection 2020. Front Immunol. 2020. PMID: 32903565 Free PMC article.
-
Using regulatory variants to detect gene-gene interactions identifies networks of genes linked to cell immortalisation.Nat Commun. 2020 Jan 17;11(1):343. doi: 10.1038/s41467-019-13762-6. Nat Commun. 2020. PMID: 31953380 Free PMC article.
References
-
- Kieff E, Rikinson A (2007) Epstein-Barr virus and its replication. Fields Virology 2603–2654.
-
- Kashuba E, Yurchenko M, Yenamandra SP, Snopok B, Szekely L, et al. (2011) Epstein-Barr virus-encoded EBNA-5 forms trimolecular protein complexes with MDM2 and p53 and inhibits the transactivating function of p53. Int J Cancer 128: 817–825. - PubMed
-
- Klein G, Klein E, Kashuba E (2010) Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem Biophys Res Commun 396: 67–73. - PubMed
-
- Kashuba E, Kashuba V, Pokrovskaja K, Klein G, Szekely L (2000) Epstein-Barr virus encoded nuclear protein EBNA-3 binds XAP-2, a protein associated with Hepatitis B virus X antigen. Oncogene 19: 1801–1806. - PubMed
-
- Kashuba EV, Gradin K, Isaguliants M, Szekely L, Poellinger L, et al. (2006) Regulation of transactivation function of the aryl hydrocarbon receptor by the Epstein-Barr virus-encoded EBNA-3 protein. J Biol Chem 281: 1215–1223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

