The effect of mass immunisation campaigns and new oral poliovirus vaccines on the incidence of poliomyelitis in Pakistan and Afghanistan, 2001-11: a retrospective analysis
- PMID: 22766207
- PMCID: PMC3418593
- DOI: 10.1016/S0140-6736(12)60648-5
The effect of mass immunisation campaigns and new oral poliovirus vaccines on the incidence of poliomyelitis in Pakistan and Afghanistan, 2001-11: a retrospective analysis
Abstract
Background: Pakistan and Afghanistan are two of the three remaining countries yet to interrupt wild-type poliovirus transmission. The increasing incidence of poliomyelitis in these countries during 2010-11 led the Executive Board of WHO in January, 2012, to declare polio eradication a "programmatic emergency for global public health". We aimed to establish why incidence is rising in these countries despite programme innovations including the introduction of new vaccines.
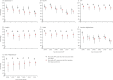
Methods: We did a matched case-control analysis based on a database of 46,977 children aged 0-14 years with onset of acute flaccid paralysis between Jan 1, 2001, and Dec 31, 2011. The vaccination history of children with poliomyelitis was compared with that of children with acute flaccid paralysis due to other causes to estimate the clinical effectiveness of oral poliovirus vaccines (OPVs) in Afghanistan and Pakistan by conditional logistic regression. We estimated vaccine coverage and serotype-specific vaccine-induced population immunity in children aged 0-2 years and assessed their association with the incidence of poliomyelitis over time in seven regions of Afghanistan and Pakistan.
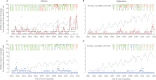
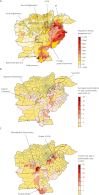
Findings: Between Jan 1, 2001, and Dec 31, 2011, there were 883 cases of serotype 1 poliomyelitis (710 in Pakistan and 173 in Afghanistan) and 272 cases of poliomyelitis serotype 3 (216 in Pakistan and 56 in Afghanistan). The estimated clinical effectiveness of a dose of trivalent OPV against serotype 1 poliomyelitis was 12·5% (95% CI 5·6-18·8) compared with 34·5% (16·1-48·9) for monovalent OPV (p=0·007) and 23·4% (10·4-34·6) for bivalent OPV (p=0·067). Bivalent OPV was non-inferior compared with monovalent OPV (p=0·21). Vaccination coverage decreased during 2006-11 in the Federally Administered Tribal Areas (FATA), Balochistan, and Khyber Pakhtunkhwa in Pakistan and in southern Afghanistan. Although partially mitigated by the use of more effective vaccines, these decreases in coverage resulted in lower vaccine-induced population immunity to poliovirus serotype 1 in FATA and Balochistan and associated increases in the incidence of poliomyelitis.
Interpretation: The effectiveness of bivalent OPV is comparable with monovalent OPV and can therefore be used in eradicating serotype 1 poliomyelitis whilst minimising the risks of serotype 3 outbreaks. However, decreases in vaccination coverage in parts of Pakistan and southern Afghanistan have severely limited the effect of this vaccine.
Funding: Poliovirus Research subcommittee of WHO, Royal Society, and Medical Research Council.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Polio vaccines and the eradication of poliomyelitis.Lancet. 2012 Aug 4;380(9840):454-5. doi: 10.1016/S0140-6736(12)60921-0. Epub 2012 Jul 4. Lancet. 2012. PMID: 22766208 No abstract available.
-
The final push for polio eradication?Lancet. 2012 Aug 4;380(9840):460-2. doi: 10.1016/s0140-6736(12)61279-3. Lancet. 2012. PMID: 22870511 No abstract available.
-
Polio vaccination in Pakistan.Lancet. 2012 Nov 10;380(9854):1645; author reply 1645-6. doi: 10.1016/S0140-6736(12)61944-8. Lancet. 2012. PMID: 23141611 No abstract available.
Similar articles
-
Population Immunity against Serotype-2 Poliomyelitis Leading up to the Global Withdrawal of the Oral Poliovirus Vaccine: Spatio-temporal Modelling of Surveillance Data.PLoS Med. 2016 Oct 4;13(10):e1002140. doi: 10.1371/journal.pmed.1002140. eCollection 2016 Oct. PLoS Med. 2016. PMID: 27701425 Free PMC article.
-
Key issues in the persistence of poliomyelitis in Nigeria: a case-control study.Lancet Glob Health. 2014 Feb;2(2):e90-7. doi: 10.1016/S2214-109X(13)70168-2. Epub 2014 Jan 23. Lancet Glob Health. 2014. PMID: 25104665
-
Estimation of oral poliovirus vaccine effectiveness in Afghanistan, 2010-2020.Vaccine. 2021 Oct 8;39(42):6250-6255. doi: 10.1016/j.vaccine.2021.09.020. Epub 2021 Sep 16. Vaccine. 2021. PMID: 34538696 Free PMC article.
-
Wild and vaccine-derived poliovirus circulation, and implications for polio eradication.Epidemiol Infect. 2017 Feb;145(3):413-419. doi: 10.1017/S0950268816002569. Epub 2016 Nov 21. Epidemiol Infect. 2017. PMID: 27866483 Free PMC article. Review.
-
Epidemiology of poliomyelitis--options and update.Vaccine. 2008 Oct 23;26(45):5767-73. doi: 10.1016/j.vaccine.2008.07.101. Epub 2008 Aug 26. Vaccine. 2008. PMID: 18755232 Review.
Cited by
-
Predictive spatial risk model of poliovirus to aid prioritization and hasten eradication in Nigeria.BMC Med. 2014 Jun 4;12:92. doi: 10.1186/1741-7015-12-92. BMC Med. 2014. PMID: 24894345 Free PMC article.
-
Factors Associated with Vaccine Refusal (Polio and Routine Immunization) in High-Risk Areas of Pakistan: A Matched Case-Control Study.Vaccines (Basel). 2023 May 5;11(5):947. doi: 10.3390/vaccines11050947. Vaccines (Basel). 2023. PMID: 37243051 Free PMC article.
-
Effect of Population Partitioning on the Probability of Silent Circulation of Poliovirus.Bull Math Biol. 2022 May 4;84(6):62. doi: 10.1007/s11538-022-01014-6. Bull Math Biol. 2022. PMID: 35507206 Free PMC article.
-
Risk factors and short-term projections for serotype-1 poliomyelitis incidence in Pakistan: A spatiotemporal analysis.PLoS Med. 2017 Jun 12;14(6):e1002323. doi: 10.1371/journal.pmed.1002323. eCollection 2017 Jun. PLoS Med. 2017. PMID: 28604777 Free PMC article.
-
Impact of Supplementary Immunization Activities using Novel Oral Polio Vaccine Type 2 during a Large outbreak of Circulating Vaccine-Derived Poliovirus in Nigeria.J Infect Dis. 2024 Mar 14;229(3):805-812. doi: 10.1093/infdis/jiad222. J Infect Dis. 2024. PMID: 37357964 Free PMC article.
References
-
- WHO Polio case count. http://apps.who.int/immunization_monitoring/en/diseases/poliomyelitis/ca... (accessed Feb 20, 2012).
-
- Independent Monitoring Board of the Global Polio Eradication Initiative Ten months and counting, February 2012. http://www.polioeradication.org/Portals/0/Document/Aboutus/Governance/IM... (accessed April 6, 2012).
-
- WHO Outbreak news. Confirmed international spread of wild poliovirus from Pakistan. Wkly Epidemiol Rec. 2011;86:437–438. - PubMed
-
- Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–939. - PubMed
-
- Caceres VM, Sutter RW. Sabin monovalent oral polio vaccines: review of past experiences and their potential use after polio eradication. Clin Infect Dis. 2001;33:531–541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

