Cellular Factors Involved in HTLV-1 Entry and Pathogenicit
- PMID: 22737146
- PMCID: PMC3380293
- DOI: 10.3389/fmicb.2012.00222
Cellular Factors Involved in HTLV-1 Entry and Pathogenicit
Abstract
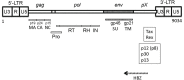
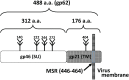
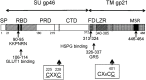
Human T cell leukemia virus type 1 (HTLV-1) is the causative agent of adult T cell leukemia (ATL) and HTLV-1 - associated myelopathy and tropical spastic paraparesis (HAM/TSP). HTLV-1 has a preferential tropism for CD4 T cells in healthy carriers and ATL patients, while both CD4 and CD8 T cells serve as viral reservoirs in HAM/TSP patients. HTLV-1 has also been detected other cell types, including monocytes, endothelial cells, and dendritic cells. In contrast to the limited cell tropism of HTLV-1 in vivo, the HTLV receptor appears to be expressed in almost all human or animal cell lines. It remains to be examined whether this cell tropism is determined by host factors or by HTLV-1 heterogeneity. Unlike most retroviruses, cell-free virions of HTLV-1 are very poorly infectious. The lack of completely HTLV-1-resistant cells and the low infectivity of HTLV-1 have hampered research on the HTLV entry receptor. Entry of HTLV-1 into target cells is thought to involve interactions between the env (Env) glycoproteins, a surface glycoprotein (surface unit), and a transmembrane glycoprotein. Recent studies have shown that glucose transporter GLUT1, heparan sulfate proteoglycans (HSPGs), and neuropilin-1 (NRP-1) are the three proteins important for the entry of HTLV-1. Studies using adherent cell lines have shown that GLUT1 can function as a receptor for HTLV. HSPGs are required for efficient entry of HTLV-1 into primary CD4 T cells. NRP-1 is expressed in most established cell lines. Further studies have shown that these three molecules work together to promote HTLV-1 binding to cells and fusion of viral and cell membranes. The virus could first contact with HSPGs and then form complexes with NRP-1, followed by association with GLUT1. It remains to be determined whether these three molecules can explain HTLV-1 cell tropism. It also remains to be more definitively proven that these molecules are sufficient to permit HTLV-1 entry into completely HTLV-1-resistant cells.
Keywords: Env isomerization; Env proteins; cell tropism; epidemiology; glucose transporter GLUT1; heparan sulfate; neuropilin-1; syncytia formation.
Figures
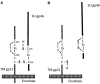
Similar articles
-
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complexes to enter T cells.J Virol. 2006 Sep;80(17):8291-302. doi: 10.1128/JVI.00389-06. J Virol. 2006. PMID: 16912281 Free PMC article.
-
Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects.Rev Neurol (Paris). 2012 Mar;168(3):257-69. doi: 10.1016/j.neurol.2011.12.006. Epub 2012 Mar 7. Rev Neurol (Paris). 2012. PMID: 22405461 Review.
-
Separate cellular localizations of human T-lymphotropic virus 1 (HTLV-1) Env and glucose transporter type 1 (GLUT1) are required for HTLV-1 Env-mediated fusion and infection.J Virol. 2015 Jan;89(1):502-11. doi: 10.1128/JVI.02686-14. Epub 2014 Oct 22. J Virol. 2015. PMID: 25339765 Free PMC article.
-
The receptor complex associated with human T-cell lymphotropic virus type 3 (HTLV-3) Env-mediated binding and entry is distinct from, but overlaps with, the receptor complexes of HTLV-1 and HTLV-2.J Virol. 2009 May;83(10):5244-55. doi: 10.1128/JVI.02285-08. Epub 2009 Mar 11. J Virol. 2009. PMID: 19279090 Free PMC article.
-
HTLV envelopes and their receptor GLUT1, the ubiquitous glucose transporter: a new vision on HTLV infection?Front Biosci. 2004 Sep 1;9:3218-41. doi: 10.2741/1474. Front Biosci. 2004. PMID: 15353351 Review.
Cited by
-
Human T-lymphotropic virus type 1 (HTLV-1) and cellular immune response in HTLV-1-associated myelopathy/tropical spastic paraparesis.J Neurovirol. 2020 Oct;26(5):652-663. doi: 10.1007/s13365-020-00881-w. Epub 2020 Jul 23. J Neurovirol. 2020. PMID: 32705480 Free PMC article. Review.
-
Pathways of cell-cell transmission of HTLV-1.Front Microbiol. 2012 Oct 24;3:378. doi: 10.3389/fmicb.2012.00378. eCollection 2012. Front Microbiol. 2012. PMID: 23109932 Free PMC article.
-
Receptor usage and the pathogenesis in acute and chronic virus infections.Front Microbiol. 2012 Aug 8;3:289. doi: 10.3389/fmicb.2012.00289. eCollection 2012. Front Microbiol. 2012. PMID: 23024639 Free PMC article. No abstract available.
-
Microbial Biofilms: Human T-cell Leukemia Virus Type 1 First in Line for Viral Biofilm but Far Behind Bacterial Biofilms.Front Microbiol. 2020 Sep 15;11:2041. doi: 10.3389/fmicb.2020.02041. eCollection 2020. Front Microbiol. 2020. PMID: 33042035 Free PMC article. Review.
-
Polyploid Giant Cancer Cells, a Hallmark of Oncoviruses and a New Therapeutic Challenge.Front Oncol. 2020 Oct 14;10:567116. doi: 10.3389/fonc.2020.567116. eCollection 2020. Front Oncol. 2020. PMID: 33154944 Free PMC article. Review.
References
-
- Abe M., Uchihashi K., Kazuto T., Osaka A., Yanagihara K., Tsukasaki K., Hasegawa H., Yamada Y., Kamihira S. (2008). Foxp3 expression on normal and leukemic CD4+CD25+ T cells implicated in human T-cell leukemia virus type-1 is inconsistent with Treg cells. Eur. J. Haematol. 81, 209–21710.1111/j.1600-0609.2008.01105.x - DOI - PubMed
-
- Akagi T., Takeda I., Oka T., Ohtsuki Y., Yano S., Miyoshi I. (1985). Experimental infection of rabbits with human T-cell leukemia virus type 1. Jpn. J. Cancer Res. 76, 86–94 - PubMed
-
- Albrecht B., Collins N. D., Burniston M. T., Nisbet J. W., Ratner L., Green P. L., Lairmore M. D. (2000). Human T-lymphotropic virus type 1 open reading frame I p12(I) is required for efficient viral infectivity in primary lymphocytes. J. Virol. 74, 9828–983510.1128/JVI.74.2.1033-1037.2000 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

