An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation
- PMID: 22723300
- PMCID: PMC3572231
- DOI: 10.1161/CIRCRESAHA.112.269399
An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation
Abstract
Rationale: Lymphatic vasculature plays important roles in tissue fluid homeostasis maintenance and in the pathology of human diseases. Yet, the molecular mechanisms that control lymphatic vessel maturation remain largely unknown.
Objective: We analyzed the gene expression profiles of ex vivo isolated lymphatic endothelial cells to identify novel lymphatic vessel expressed genes and we investigated the role of semaphorin 3A (Sema3A) and neuropilin-1 (Nrp-1) in lymphatic vessel maturation and function.
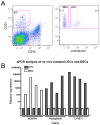
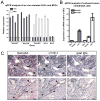
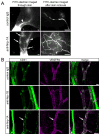
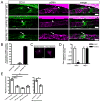
Methods and results: Lymphatic and blood vascular endothelial cells from mouse intestine were isolated using fluorescence-activated cell sorting, and transcriptional profiling was performed. We found that the axonal guidance molecules Sema3A and Sema3D were highly expressed by lymphatic vessels. Importantly, we found that the semaphorin receptor Nrp-1 is expressed on the perivascular cells of the collecting lymphatic vessels. Treatment of mice in utero (E12.5-E16.5) with an antibody that blocks Sema3A binding to Nrp-1 but not with an antibody that blocks VEGF-A binding to Nrp-1 resulted in a complex phenotype of impaired lymphatic vessel function, enhanced perivascular cell coverage, and abnormal lymphatic vessel and valve morphology.
Conclusions: Together, these results reveal an unanticipated role of Sema3A-Nrp-1 signaling in the maturation of the lymphatic vascular network likely via regulating the perivascular cell coverage of the vessels thus affecting lymphatic vessel function and lymphatic valve development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation.Circ Res. 2012 Aug 3;111(4):437-45. doi: 10.1161/CIRCRESAHA.112.269316. Epub 2012 Jun 21. Circ Res. 2012. PMID: 22723296 Free PMC article.
-
The role of neuropilin-1/semaphorin 3A signaling in lymphatic vessel development and maturation.Adv Anat Embryol Cell Biol. 2014;214:143-52. doi: 10.1007/978-3-7091-1646-3_11. Adv Anat Embryol Cell Biol. 2014. PMID: 24276892 Review.
-
Endothelial cell-derived semaphorin 3A inhibits filopodia formation by blood vascular tip cells.Development. 2016 Feb 15;143(4):589-94. doi: 10.1242/dev.127670. Development. 2016. PMID: 26884395
-
Molecular controls of lymphatic VEGFR3 signaling.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):421-9. doi: 10.1161/ATVBAHA.114.304881. Epub 2014 Dec 18. Arterioscler Thromb Vasc Biol. 2015. PMID: 25524775 Free PMC article.
-
Review of the function of SEMA3A in lymphatic vessel maturation and its potential as a candidate gene for lymphedema: Analysis of three families with rare causative variants.Lymphology. 2020;53(2):63-75. Lymphology. 2020. PMID: 33190429 Review.
Cited by
-
Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation.Blood. 2013 Jul 25;122(4):598-607. doi: 10.1182/blood-2012-12-472142. Epub 2013 Jun 5. Blood. 2013. PMID: 23741013 Free PMC article.
-
YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling.Development. 2020 Dec 13;147(23):dev195453. doi: 10.1242/dev.195453. Development. 2020. PMID: 33060128 Free PMC article.
-
Toward Characterizing Lymphatic Vasculature in the Mammary Gland During Normal Development and Tumor-Associated Remodeling.J Mammary Gland Biol Neoplasia. 2024 Jan 13;29(1):1. doi: 10.1007/s10911-023-09554-w. J Mammary Gland Biol Neoplasia. 2024. PMID: 38218743 Free PMC article. Review.
-
Lymphatic Vasculature in Energy Homeostasis and Obesity.Front Physiol. 2020 Jan 22;11:3. doi: 10.3389/fphys.2020.00003. eCollection 2020. Front Physiol. 2020. PMID: 32038308 Free PMC article. Review.
-
Lymphatic System and the Kidney: From Lymphangiogenesis to Renal Inflammation and Fibrosis Development.Int J Mol Sci. 2024 Mar 1;25(5):2853. doi: 10.3390/ijms25052853. Int J Mol Sci. 2024. PMID: 38474100 Free PMC article. Review.
References
-
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. - PubMed
-
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by vegf-c promotes breast cancer metastasis. Nat Med. 2001;7:192–198. - PubMed
-
- Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in vegf-a transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. - PubMed
-
- Szuba A, Rockson SG. Lymphedema: Anatomy, physiology and pathogenesis. Vasc Med. 1997;2:321–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

