Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation
- PMID: 22723296
- PMCID: PMC3861899
- DOI: 10.1161/CIRCRESAHA.112.269316
Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation
Abstract
Rationale: The lymphatic vasculature plays a major role in fluid homeostasis, absorption of dietary lipids, and immune surveillance. Fluid transport depends on the presence of intraluminal valves within lymphatic collectors. Defective formation of lymphatic valves leads to lymphedema, a progressive and debilitating condition for which curative treatments are currently unavailable. How lymphatic valve formation is regulated remains largely unknown.
Objective: We investigated if the repulsive axon guidance molecule Semaphorin3A (Sema3A) plays a role in lymphatic valve formation.
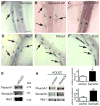
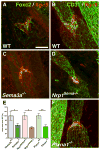
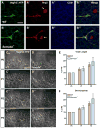
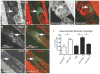
Methods and results: We show that Sema3A mRNA is expressed in lymphatic vessels and that Sema3A protein binds to lymphatic valves expressing the Neuropilin-1 (Nrp1) and PlexinA1 receptors. Using mouse knockout models, we show that Sema3A is selectively required for lymphatic valve formation, via interaction with Nrp1 and PlexinA1. Sema3a(-/-) mice exhibit defects in lymphatic valve formation, which are not due to abnormal lymphatic patterning or sprouting, and mice carrying a mutation in the Sema3A binding site of Nrp1, or deficient for Plxna1, develop lymphatic valve defects similar to those seen in Sema3a(-/-) mice.
Conclusions: Our data demonstrate an essential direct function of Sema3A-Nrp1-PlexinA1 signaling in lymphatic valve formation.
Figures

Similar articles
-
An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation.Circ Res. 2012 Aug 3;111(4):426-36. doi: 10.1161/CIRCRESAHA.112.269399. Epub 2012 Jun 20. Circ Res. 2012. PMID: 22723300 Free PMC article.
-
Adenosine A2A receptor (A2AR) stimulation modulates expression of semaphorins 4D and 3A, regulators of bone homeostasis.FASEB J. 2018 Jul;32(7):3487-3501. doi: 10.1096/fj.201700217R. Epub 2018 Feb 2. FASEB J. 2018. PMID: 29394106 Free PMC article.
-
NRP1 and NRP2 cooperate to regulate gangliogenesis, axon guidance and target innervation in the sympathetic nervous system.Dev Biol. 2012 Sep 15;369(2):277-85. doi: 10.1016/j.ydbio.2012.06.026. Epub 2012 Jul 10. Dev Biol. 2012. PMID: 22790009 Free PMC article.
-
Review of the function of SEMA3A in lymphatic vessel maturation and its potential as a candidate gene for lymphedema: Analysis of three families with rare causative variants.Lymphology. 2020;53(2):63-75. Lymphology. 2020. PMID: 33190429 Review.
-
The role of neuropilin-1/semaphorin 3A signaling in lymphatic vessel development and maturation.Adv Anat Embryol Cell Biol. 2014;214:143-52. doi: 10.1007/978-3-7091-1646-3_11. Adv Anat Embryol Cell Biol. 2014. PMID: 24276892 Review.
Cited by
-
Involvement of neuronal factors in tumor angiogenesis and the shaping of the cancer microenvironment.Front Immunol. 2024 Feb 5;15:1284629. doi: 10.3389/fimmu.2024.1284629. eCollection 2024. Front Immunol. 2024. PMID: 38375479 Free PMC article. Review.
-
Lymphatic pumping: mechanics, mechanisms and malfunction.J Physiol. 2016 Oct 15;594(20):5749-5768. doi: 10.1113/JP272088. Epub 2016 Aug 2. J Physiol. 2016. PMID: 27219461 Free PMC article. Review.
-
Semaphorin 3E/PlexinD1 signaling is required for cardiac ventricular compaction.JCI Insight. 2019 Aug 22;4(16):e125908. doi: 10.1172/jci.insight.125908. JCI Insight. 2019. PMID: 31434798 Free PMC article.
-
Non-coding RNAs as Regulators of Lymphangiogenesis in Lymphatic Development, Inflammation, and Cancer Metastasis.Front Oncol. 2019 Sep 20;9:916. doi: 10.3389/fonc.2019.00916. eCollection 2019. Front Oncol. 2019. PMID: 31616631 Free PMC article. Review.
-
Vascular and lymphatic regulation of gastrointestinal function and disease risk.Biochim Biophys Acta Mol Cell Biol Lipids. 2022 Nov;1867(11):159207. doi: 10.1016/j.bbalip.2022.159207. Epub 2022 Jul 23. Biochim Biophys Acta Mol Cell Biol Lipids. 2022. PMID: 35882297 Free PMC article. Review.
References
-
- Foldi M. Remarks concerning the consensus document (CD) of the International Society of Lymphology: “The diagnosis and treatment of peripheral lymphedema”. Lymphology. 2004;37:168–173. - PubMed
-
- Kosco-Vilbois M, Meyer-Hermann M. The 16th International Conference on Lymphatic Tissues and Germinal Centers in Immune Responses. Eur J Immunol. 2009;39:2311–2312. - PubMed
-
- Sapin MR. Lymphatic system and its significance in immune processes. Morfologiia. 2007;131:18–22. - PubMed
-
- Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. - PubMed
Methods references
-
- Moyon D, Pardanaud L, Yuan L, Bréant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. - PubMed
-
- Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas J-L, Koch AW, Alitalo K, Eichmann A, Bagri A. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. The Journal of Cell Biology. 2010;188:115–130. - PMC - PubMed
-
- Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

