Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer
- PMID: 22698406
- PMCID: PMC3575028
- DOI: 10.1016/j.ccr.2012.04.025
Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer
Abstract
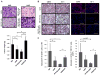
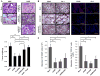
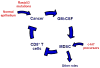
Cancer-associated inflammation is thought to be a barrier to immune surveillance, particularly in pancreatic ductal adenocarcinoma (PDA). Gr-1(+) CD11b(+) cells are a key feature of cancer inflammation in PDA, but remain poorly understood. Using a genetically engineered mouse model of PDA, we show that tumor-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) is necessary and sufficient to drive the development of Gr-1(+) CD11b(+) cells that suppressed antigen-specific T cells. In vivo, abrogation of tumor-derived GM-CSF inhibited the recruitment of Gr-1(+) CD11b(+) cells to the tumor microenvironment and blocked tumor development-a finding that was dependent on CD8(+) T cells. In humans, PDA tumor cells prominently expressed GM-CSF in vivo. Thus, tumor-derived GM-CSF is an important regulator of inflammation and immune suppression within the tumor microenvironment.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
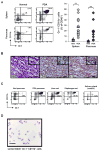
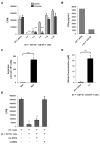
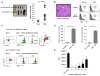
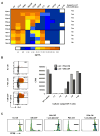

Comment in
-
Silencing the killers: paracrine immune suppression in pancreatic cancer.Cancer Cell. 2012 Jun 12;21(6):715-6. doi: 10.1016/j.ccr.2012.05.029. Cancer Cell. 2012. PMID: 22698396
-
Pancreatic cancer: The role of GM-CSF in pancreatic cancer unveiled.Nat Rev Gastroenterol Hepatol. 2012 Aug;9(8):426. doi: 10.1038/nrgastro.2012.127. Epub 2012 Jun 26. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22733349 No abstract available.
-
Suppressing the rejection of pancreatic tumours.Nat Rev Cancer. 2012 Jul 19;12(8):510-1. doi: 10.1038/nrc3329. Nat Rev Cancer. 2012. PMID: 22810812 No abstract available.
-
Tumour immunology: Suppressing the rejection of pancreatic tumours.Nat Rev Immunol. 2012 Jul 20;12(8):555. doi: 10.1038/nri3267. Nat Rev Immunol. 2012. PMID: 22814510 No abstract available.
Similar articles
-
Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia.Cancer Cell. 2012 Jun 12;21(6):836-47. doi: 10.1016/j.ccr.2012.04.024. Cancer Cell. 2012. PMID: 22698407 Free PMC article.
-
Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer.Cancer Res. 2015 Jul 1;75(13):2629-40. doi: 10.1158/0008-5472.CAN-14-2921. Epub 2015 May 7. Cancer Res. 2015. PMID: 25952647
-
CD73 induces GM-CSF/MDSC-mediated suppression of T cells to accelerate pancreatic cancer pathogenesis.Oncogene. 2022 Feb;41(7):971-982. doi: 10.1038/s41388-021-02132-6. Epub 2022 Jan 10. Oncogene. 2022. PMID: 35001076 Free PMC article.
-
T-cell programming in pancreatic adenocarcinoma: a review.Cancer Gene Ther. 2017 Mar;24(3):106-113. doi: 10.1038/cgt.2016.66. Epub 2016 Dec 2. Cancer Gene Ther. 2017. PMID: 27910859 Review.
-
Tumor-Derived Myeloid Cell Chemoattractants and T Cell Exclusion in Pancreatic Cancer.Front Immunol. 2020 Nov 10;11:605619. doi: 10.3389/fimmu.2020.605619. eCollection 2020. Front Immunol. 2020. PMID: 33304355 Free PMC article. Review.
Cited by
-
Convergent inducers and effectors of T cell paralysis in the tumour microenvironment.Nat Rev Cancer. 2025 Jan;25(1):41-58. doi: 10.1038/s41568-024-00761-z. Epub 2024 Oct 24. Nat Rev Cancer. 2025. PMID: 39448877 Review.
-
Granulocytes and Cells of Granulocyte Origin-The Relevant Players in Colorectal Cancer.Int J Mol Sci. 2021 Apr 6;22(7):3801. doi: 10.3390/ijms22073801. Int J Mol Sci. 2021. PMID: 33917620 Free PMC article. Review.
-
How Tumor Cell Dedifferentiation Drives Immune Evasion and Resistance to Immunotherapy.Cancer Res. 2020 Oct 1;80(19):4037-4041. doi: 10.1158/0008-5472.CAN-20-1420. Epub 2020 Jun 18. Cancer Res. 2020. PMID: 32554552 Free PMC article. Review.
-
Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+) ;LSL-Trp53(R172H/+) ;Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery.Curr Protoc Pharmacol. 2016 Jun 1;73:14.39.1-14.39.20. doi: 10.1002/cpph.2. Curr Protoc Pharmacol. 2016. PMID: 27248578 Free PMC article.
-
Therapeutic Targeting of the Tumour Microenvironment in Metastatic Colorectal Cancer.Int J Mol Sci. 2021 Feb 19;22(4):2067. doi: 10.3390/ijms22042067. Int J Mol Sci. 2021. PMID: 33669775 Free PMC article. Review.
References
-
- Bellone G, Carbone A, Smirne C, Scirelli T, Buffolino A, Novarino A, Stacchini A, Bertetto O, Palestro G, Sorio C, et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J Immunol. 2006;177:3448–3460. - PubMed
-
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

