Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to
- PMID: 22683661
- PMCID: PMC3384435
- DOI: 10.18632/aging.100461
Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to
Abstract
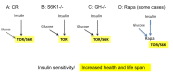
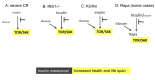
Calorie restriction (CR), which deactivates the nutrient-sensing mTOR pathway, slows down aging and prevents age-related diseases such as type II diabetes. Compared with CR, rapamycin more efficiently inhibits mTOR. Noteworthy, severe CR and starvation cause a reversible condition known as "starvation diabetes." As was already discussed, chronic administration of rapamycin can cause a similar condition in some animal models. A recent paper published in Science reported that chronic treatment with rapamycin causes a diabetes-like condition in mice by indirectly inhibiting mTOR complex 2. Here I introduce the notion of benevolent diabetes and discuss whether starvation-like effects of chronic high dose treatment with rapamycin are an obstacle for its use as an anti-aging drug.
Conflict of interest statement
The author of this manuscript has no conflict of interest to declare.
Figures

Similar articles
-
Koschei the immortal and anti-aging drugs.Cell Death Dis. 2014 Dec 4;5(12):e1552. doi: 10.1038/cddis.2014.520. Cell Death Dis. 2014. PMID: 25476900 Free PMC article. Review.
-
Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans).Cell Cycle. 2010 Feb 15;9(4):683-8. doi: 10.4161/cc.9.4.10766. Epub 2010 Mar 2. Cell Cycle. 2010. PMID: 20139716
-
Rapamycin-induced glucose intolerance: hunger or starvation diabetes.Cell Cycle. 2011 Dec 15;10(24):4217-24. doi: 10.4161/cc.10.24.18595. Epub 2011 Dec 15. Cell Cycle. 2011. PMID: 22157190
-
The use of calorie restriction mimetics to study aging.Methods Mol Biol. 2013;1048:95-107. doi: 10.1007/978-1-62703-556-9_8. Methods Mol Biol. 2013. PMID: 23929100
-
Rapalogs in cancer prevention: anti-aging or anticancer?Cancer Biol Ther. 2012 Dec;13(14):1349-54. doi: 10.4161/cbt.22859. Epub 2012 Nov 14. Cancer Biol Ther. 2012. PMID: 23151465 Free PMC article. Review.
Cited by
-
Relationship between four visceral obesity indices and prediabetes and diabetes: a cross-sectional study in Dalian, China.BMC Endocr Disord. 2024 Sep 18;24(1):191. doi: 10.1186/s12902-024-01718-x. BMC Endocr Disord. 2024. PMID: 39294627 Free PMC article.
-
Beneficial effects of novel antagonists of GHRH in different models of Alzheimer's disease.Aging (Albany NY). 2012 Nov;4(11):755-67. doi: 10.18632/aging.100504. Aging (Albany NY). 2012. PMID: 23211425 Free PMC article.
-
Gerosuppression in confluent cells.Aging (Albany NY). 2014 Dec;6(12):1010-8. doi: 10.18632/aging.100714. Aging (Albany NY). 2014. PMID: 25585637 Free PMC article.
-
Rapamycin extends life- and health span because it slows aging.Aging (Albany NY). 2013 Aug;5(8):592-8. doi: 10.18632/aging.100591. Aging (Albany NY). 2013. PMID: 23934728 Free PMC article.
-
Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives.Front Pharmacol. 2016 Oct 25;7:395. doi: 10.3389/fphar.2016.00395. eCollection 2016. Front Pharmacol. 2016. PMID: 27826244 Free PMC article. Review.
References
-
- Hughes KJ, Kennedy BK. Cell biology. Rapamycin paradox resolved. Science. 2012;335:1578–1579. - PubMed
-
- Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–957. - PubMed
-
- Chang GR, Wu YY, Chiu YS, Chen WY, Liao JW, Hsu HM, Chao TH, Hung SW, Mao FC. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105:188–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

