The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi's sarcoma–associated herpesvirus
- PMID: 22635007
- PMCID: PMC3645317
- DOI: 10.1038/nm.2805
The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi's sarcoma–associated herpesvirus
Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of Kaposi's sarcoma(1), a highly vascularized tumor originating from lymphatic endothelial cells, and of at least two different B cell malignancies(2,3). A dimeric complex formed by the envelope glycoproteins H and L (gH-gL) is required for entry of herpesviruses into host cells(4). We show that the ephrin receptor tyrosine kinase A2 (EphA2) is a cellular receptor for KSHV gH-gL. EphA2 co-precipitated with both gH-gL and KSHV virions. Infection of human epithelial cells with a GFP-expressing recombinant KSHV strain, as measured by FACS analysis, was increased upon overexpression of EphA2. Antibodies against EphA(2) and siRNAs directed against EphA2 inhibited infection of endothelial cells. Pretreatment of KSHV with soluble EphA2 resulted in inhibition of KSHV infection by up to 90%. This marked reduction of KSHV infection was seen with all the different epithelial and endothelial cells used in this study. Similarly, pretreating epithelial or endothelial cells with the soluble EphA2 ligand ephrinA4 impaired KSHV infection. Deletion of the gene encoding EphA2 essentially abolished KSHV infection of mouse endothelial cells. Binding of gH-gL to EphA2 triggered EphA2 phosphorylation and endocytosis, a major pathway of KSHV entry(5,6). Quantitative RT-PCR and in situ histochemistry revealed a close correlation between KSHV infection and EphA2 expression both in cultured cells derived from human Kaposi's sarcoma lesions or unaffected human lymphatic endothelium, and in situ in Kaposi's sarcoma specimens, respectively. Taken together, our results identify EphA2, a tyrosine kinase with known functions in neovascularization and oncogenesis, as an entry receptor for KSHV.
Figures


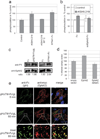
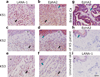
Comment in
-
Tumour virology. Entry requirements.Nat Rev Cancer. 2012 Jun 22;12(7):450. doi: 10.1038/nrc3312. Nat Rev Cancer. 2012. PMID: 22722396
Similar articles
-
Ephrin Receptor A4 is a New Kaposi's Sarcoma-Associated Herpesvirus Virus Entry Receptor.mBio. 2019 Feb 19;10(1):e02892-18. doi: 10.1128/mBio.02892-18. mBio. 2019. PMID: 30782663 Free PMC article.
-
Epstein-Barr Virus gH/gL and Kaposi's Sarcoma-Associated Herpesvirus gH/gL Bind to Different Sites on EphA2 To Trigger Fusion.J Virol. 2020 Oct 14;94(21):e01454-20. doi: 10.1128/JVI.01454-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32847853 Free PMC article.
-
EphA7 Functions as Receptor on BJAB Cells for Cell-to-Cell Transmission of the Kaposi's Sarcoma-Associated Herpesvirus and for Cell-Free Infection by the Related Rhesus Monkey Rhadinovirus.J Virol. 2019 Jul 17;93(15):e00064-19. doi: 10.1128/JVI.00064-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31118261 Free PMC article.
-
KSHV Entry and Trafficking in Target Cells-Hijacking of Cell Signal Pathways, Actin and Membrane Dynamics.Viruses. 2016 Nov 14;8(11):305. doi: 10.3390/v8110305. Viruses. 2016. PMID: 27854239 Free PMC article. Review.
-
Potential entry receptors for human γ-herpesvirus into epithelial cells: A plausible therapeutic target for viral infections.Tumour Virus Res. 2021 Dec;12:200227. doi: 10.1016/j.tvr.2021.200227. Epub 2021 Nov 18. Tumour Virus Res. 2021. PMID: 34800753 Free PMC article. Review.
Cited by
-
Stuck in the middle: structural insights into the role of the gH/gL heterodimer in herpesvirus entry.Curr Opin Virol. 2013 Feb;3(1):13-9. doi: 10.1016/j.coviro.2012.10.005. Epub 2012 Oct 26. Curr Opin Virol. 2013. PMID: 23107819 Free PMC article. Review.
-
The extracellular interactome of the human adenovirus family reveals diverse strategies for immunomodulation.Nat Commun. 2016 May 5;7:11473. doi: 10.1038/ncomms11473. Nat Commun. 2016. PMID: 27145901 Free PMC article.
-
Kaposi sarcoma associated herpesvirus pathogenesis (KSHV)--an update.Curr Opin Virol. 2013 Jun;3(3):238-44. doi: 10.1016/j.coviro.2013.05.012. Epub 2013 Jun 13. Curr Opin Virol. 2013. PMID: 23769237 Free PMC article. Review.
-
Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy.J Clin Invest. 2016 Sep 1;126(9):3165-75. doi: 10.1172/JCI84418. Epub 2016 Sep 1. J Clin Invest. 2016. PMID: 27584730 Free PMC article. Review.
-
Saracatinib Inhibits Middle East Respiratory Syndrome-Coronavirus Replication In Vitro.Viruses. 2018 May 24;10(6):283. doi: 10.3390/v10060283. Viruses. 2018. PMID: 29795047 Free PMC article.
References
-
- Chang Y, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995;332:1186–1191. - PubMed
-
- Soulier J, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. - PubMed
-
- Campadelli-Fiume G, et al. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev. Med. Virol. 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

