Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis
- PMID: 22629478
- PMCID: PMC3358331
- DOI: 10.1371/journal.pntd.0001657
Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis
Abstract
Background: With widespread resistance to antimonials in Visceral Leishmaniasis (VL) in the Indian subcontinent, Miltefosine (MIL) has been introduced as the first line therapy. Surveillance of MIL susceptibility in natural populations of Leishmania donovani is vital to preserve it and support the VL elimination program.
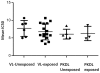
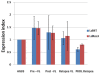
Methodology and principal findings: We measured in vitro susceptibility towards MIL and paromomycin (PMM) in L. donovani isolated from VL and PKDL, pre- and post-treatment cases, using an amastigote-macrophage model. MIL susceptibility of post-treatment isolates from cured VL cases (n = 13, mean IC(50)±SD = 2.43±1.44 µM), was comparable (p>0.05) whereas that from relapses (n = 3, mean IC(50) = 4.72±1.99 µM) was significantly higher (p = 0.04) to that of the pre-treatment group (n = 6, mean IC(50) = 1.86±0.75 µM). In PKDL, post-treatment isolates (n = 3, mean IC(50) = 16.13±2.64 µM) exhibited significantly lower susceptibility (p = 0.03) than pre-treatment isolates (n = 5, mean IC(50) = 8.63±0.94 µM). Overall, PKDL isolates (n = 8, mean IC(50) = 11.45±4.19 µM) exhibited significantly higher tolerance (p<0.0001) to MIL than VL isolates (n = 22, mean IC(50) = 2.58±1.58 µM). Point mutations in the miltefosine transporter (LdMT) and its beta subunit (LdRos3) genes previously reported in parasites with experimentally induced MIL resistance were not present in the clinical isolates. Further, the mRNA expression profile of these genes was comparable in the pre- and post-treatment isolates. Parasite isolates from VL and PKDL cases were uniformly susceptible to PMM with respective mean IC(50) = 7.05±2.24 µM and 6.18±1.51 µM.
Conclusion: The in vitro susceptibility of VL isolates remained unchanged at the end of MIL treatment; however, isolates from relapsed VL and PKDL cases had lower susceptibility than the pre-treatment isolates. PKDL isolates were more tolerant towards MIL in comparison with VL isolates. All parasite isolates were uniformly susceptible to PMM. Mutations in the LdMT and LdRos3 genes as well as changes in the expression of these genes previously correlated with experimental resistance to MIL could not be verified for the field isolates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Antimony susceptible Leishmania donovani: evidence from in vitro drug susceptibility of parasites isolated from patients of post-kala-azar dermal leishmaniasis in pre- and post-miltefosine era.Microbiol Spectr. 2024 Jun 4;12(6):e0402623. doi: 10.1128/spectrum.04026-23. Epub 2024 May 7. Microbiol Spectr. 2024. PMID: 38712926 Free PMC article.
-
Visceral and post-Kala-Azar dermal leishmaniasis isolates show significant difference in their in vitro drug susceptibility pattern.Parasitol Res. 2013 Mar;112(3):1001-9. doi: 10.1007/s00436-012-3222-1. Epub 2012 Dec 18. Parasitol Res. 2013. PMID: 23242321
-
Increased miltefosine tolerance in clinical isolates of Leishmania donovani is associated with reduced drug accumulation, increased infectivity and resistance to oxidative stress.PLoS Negl Trop Dis. 2017 Jun 2;11(6):e0005641. doi: 10.1371/journal.pntd.0005641. eCollection 2017 Jun. PLoS Negl Trop Dis. 2017. PMID: 28575060 Free PMC article.
-
Evaluating drug resistance in visceral leishmaniasis: the challenges.Parasitology. 2018 Apr;145(4):453-463. doi: 10.1017/S0031182016002031. Epub 2016 Nov 21. Parasitology. 2018. PMID: 27866478 Free PMC article. Review.
-
Post-kala-azar dermal leishmaniasis in the Indian subcontinent: A threat to the South-East Asia Region Kala-azar Elimination Programme.PLoS Negl Trop Dis. 2017 Nov 16;11(11):e0005877. doi: 10.1371/journal.pntd.0005877. eCollection 2017 Nov. PLoS Negl Trop Dis. 2017. PMID: 29145397 Free PMC article. Review.
Cited by
-
Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus Viannia species.Antimicrob Agents Chemother. 2014;58(1):144-52. doi: 10.1128/AAC.01023-13. Epub 2013 Oct 21. Antimicrob Agents Chemother. 2014. PMID: 24145529 Free PMC article.
-
Laboratory confirmed miltefosine resistant cases of visceral leishmaniasis from India.Parasit Vectors. 2017 Jan 31;10(1):49. doi: 10.1186/s13071-017-1969-z. Parasit Vectors. 2017. PMID: 28137296 Free PMC article.
-
In-Depth Quantitative Proteomics Characterization of In Vitro Selected Miltefosine Resistance in Leishmania infantum.Proteomes. 2022 Mar 31;10(2):10. doi: 10.3390/proteomes10020010. Proteomes. 2022. PMID: 35466238 Free PMC article.
-
Clinical and parasitological factors in parasite persistence after treatment and clinical cure of cutaneous leishmaniasis.PLoS Negl Trop Dis. 2017 Jul 13;11(7):e0005713. doi: 10.1371/journal.pntd.0005713. eCollection 2017 Jul. PLoS Negl Trop Dis. 2017. PMID: 28704369 Free PMC article.
-
Reverse Epidemiology: An Experimental Framework to Drive Leishmania Biomarker Discovery in situ by Functional Genetic Screening Using Relevant Animal Models.Front Cell Infect Microbiol. 2018 Sep 19;8:325. doi: 10.3389/fcimb.2018.00325. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30283743 Free PMC article. Review.
References
-
- Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, et al. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. - PubMed
-
- WHO. Expert Committee on Control of the leishmaniases. 2010. WHO Technical Report Series.
-
- WHO. 2007. Expert Committee Available: http// www.who.int/Leishmaniasis/en.
-
- Jha TK, Sundar S, Thakur CP, Felton JM, Sabin AJ, et al. A phase II dose-ranging study of sitamaquine for the treatment of visceral leishmaniasis in India. Am J Trop Med Hyg. 2005;73:1005–1011. - PubMed
-
- Olliaro PL, Guerin PJ, Gerstl S, Haaskjold AA, Rottingen JA, et al. Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980–2004. Lancet Infect Dis. 2005;5:763–774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

