Protein B5 is required on extracellular enveloped vaccinia virus for repulsion of superinfecting virions
- PMID: 22622330
- PMCID: PMC3709573
- DOI: 10.1099/vir.0.043943-0
Protein B5 is required on extracellular enveloped vaccinia virus for repulsion of superinfecting virions
Abstract
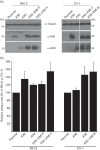
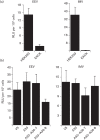
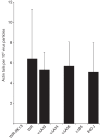
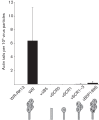
Vaccinia virus (VACV) spreads across cell monolayers fourfold faster than predicted from its replication kinetics. Early after infection, infected cells repulse some superinfecting extracellular enveloped virus (EEV) particles by the formation of actin tails from the cell surface, thereby causing accelerated spread to uninfected cells. This strategy requires the expression of two viral proteins, A33 and A36, on the surface of infected cells and upon contact with EEV this complex induces actin polymerization. Here we have studied this phenomenon further and investigated whether A33 and A36 expression in cell lines causes an increase in VACV plaque size, whether these proteins are able to block superinfection by EEV, and which protein(s) on the EEV surface are required to initiate the formation of actin tails from infected cells. Data presented show that VACV plaque size was not increased by expression of A33 and A36, and these proteins did not block entry of the majority of EEV binding to these cells. In contrast, expression of proteins A56 and K2 inhibited entry of both EEV and intracellular mature virus. Lastly, VACV protein B5 was required on EEV to induce the formation of actin tails at the surface of cells expressing A33 and A36, and B5 short consensus repeat 4 is critical for this induction.
Figures

Similar articles
-
Repulsion of superinfecting virions: a mechanism for rapid virus spread.Science. 2010 Feb 12;327(5967):873-876. doi: 10.1126/science.1183173. Epub 2010 Jan 21. Science. 2010. PMID: 20093437 Free PMC article.
-
Mutations Near the N Terminus of Vaccinia Virus G9 Protein Overcome Restrictions on Cell Entry and Syncytium Formation Imposed by the A56/K2 Fusion Regulatory Complex.J Virol. 2020 May 4;94(10):e00077-20. doi: 10.1128/JVI.00077-20. Print 2020 May 4. J Virol. 2020. PMID: 32132239 Free PMC article.
-
Acidic residues in the membrane-proximal stalk region of vaccinia virus protein B5 are required for glycosaminoglycan-mediated disruption of the extracellular enveloped virus outer membrane.J Gen Virol. 2009 Jul;90(Pt 7):1582-1591. doi: 10.1099/vir.0.009092-0. Epub 2009 Mar 4. J Gen Virol. 2009. PMID: 19264647 Free PMC article.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
-
The formation and function of extracellular enveloped vaccinia virus.J Gen Virol. 2002 Dec;83(Pt 12):2915-2931. doi: 10.1099/0022-1317-83-12-2915. J Gen Virol. 2002. PMID: 12466468 Review.
Cited by
-
Two noncompeting human neutralizing antibodies targeting MPXV B6 show protective effects against orthopoxvirus infections.Nat Commun. 2024 May 31;15(1):4660. doi: 10.1038/s41467-024-48312-2. Nat Commun. 2024. PMID: 38821921 Free PMC article.
-
Unusual features of vaccinia virus extracellular virion form neutralization resistance revealed in human antibody responses to the smallpox vaccine.J Virol. 2013 Feb;87(3):1569-85. doi: 10.1128/JVI.02152-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152530 Free PMC article.
-
Vaccinia virus protein complex F12/E2 interacts with kinesin light chain isoform 2 to engage the kinesin-1 motor complex.PLoS Pathog. 2015 Mar 11;11(3):e1004723. doi: 10.1371/journal.ppat.1004723. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25760349 Free PMC article.
-
Tagging of the vaccinia virus protein F13 with mCherry causes aberrant virion morphogenesis.J Gen Virol. 2017 Oct;98(10):2543-2555. doi: 10.1099/jgv.0.000917. J Gen Virol. 2017. PMID: 28933687 Free PMC article.
-
Host Src controls gallid alpha herpesvirus 1 intercellular spread in a cellular fatty acid metabolism-dependent manner.Virology. 2019 Nov;537:1-13. doi: 10.1016/j.virol.2019.08.011. Epub 2019 Aug 13. Virology. 2019. PMID: 31425969 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

