ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero
- PMID: 22563477
- PMCID: PMC3341399
- DOI: 10.1371/journal.pone.0036055
ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero
Abstract
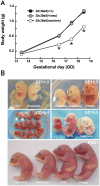
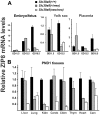
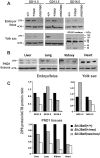
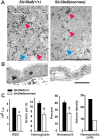
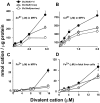
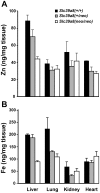
Previously this laboratory characterized Slc39a8-encoded ZIP8 as a Zn(2+)/(HCO(3)(-))(2) symporter; yet, the overall physiological importance of ZIP8 at the whole-organism level remains unclear. Herein we describe the phenotype of the hypomorphic Slc39a8(neo/neo) mouse which has retained the neomycin-resistance gene in intron 3, hence causing significantly decreased ZIP8 mRNA and protein levels in embryo, fetus, placenta, yolk sac, and several tissues of neonates. The Slc39a8(neo) allele is associated with diminished zinc and iron uptake in mouse fetal fibroblast and liver-derived cultures; consequently, Slc39a8(neo/neo) newborns exhibit diminished zinc and iron levels in several tissues. Slc39a8(neo/neo) homozygotes from gestational day(GD)-11.5 onward are pale, growth-stunted, and die between GD18.5 and 48 h postnatally. Defects include: severely hypoplastic spleen; hypoplasia of liver, kidney, lung, and lower limbs. Histologically, Slc39a8(neo/neo) neonates show decreased numbers of hematopoietic islands in yolk sac and liver. Low hemoglobin, hematocrit, red cell count, serum iron, and total iron-binding capacity confirmed severe anemia. Flow cytometry of fetal liver cells revealed the erythroid series strikingly affected in the hypomorph. Zinc-dependent 5-aminolevulinic acid dehydratase, required for heme synthesis, was not different between Slc39a8(+/+) and Slc39a8(neo/neo) offspring. To demonstrate further that the mouse phenotype is due to ZIP8 deficiency, we bred Slc39a8(+/neo) with BAC-transgenic BTZIP8-3 line (carrying three extra copies of the Slc39a8 allele); this cross generated viable Slc39a8(neo/neo)_BTZIP8-3(+/+) pups showing none of the above-mentioned congenital defects-proving Slc39a8(neo/neo) causes the described phenotype. Our study demonstrates that ZIP8-mediated zinc transport plays an unappreciated critical role during in utero and neonatal growth, organ morphogenesis, and hematopoiesis.
Conflict of interest statement
Figures

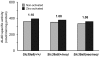
Similar articles
-
Generation of a Slc39a8 hypomorph mouse: markedly decreased ZIP8 Zn²⁺/(HCO₃⁻)₂ transporter expression.Biochem Biophys Res Commun. 2011 Jul 1;410(2):289-94. doi: 10.1016/j.bbrc.2011.05.134. Epub 2011 May 31. Biochem Biophys Res Commun. 2011. PMID: 21658371 Free PMC article.
-
In utero gene expression in the Slc39a8(neo/neo) knockdown mouse.Sci Rep. 2018 Jul 16;8(1):10703. doi: 10.1038/s41598-018-29109-y. Sci Rep. 2018. PMID: 30013175 Free PMC article.
-
SLC39A8 gene encoding a metal ion transporter: discovery and bench to bedside.Hum Genomics. 2019 Sep 14;13(Suppl 1):51. doi: 10.1186/s40246-019-0233-3. Hum Genomics. 2019. PMID: 31521203 Free PMC article. Review.
-
Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line.Am J Physiol Cell Physiol. 2007 Apr;292(4):C1523-35. doi: 10.1152/ajpcell.00409.2006. Epub 2006 Nov 15. Am J Physiol Cell Physiol. 2007. PMID: 17108009
-
Discovery of ZIP transporters that participate in cadmium damage to testis and kidney.Toxicol Appl Pharmacol. 2009 Aug 1;238(3):250-7. doi: 10.1016/j.taap.2009.02.017. Epub 2009 Mar 2. Toxicol Appl Pharmacol. 2009. PMID: 19265717 Free PMC article. Review.
Cited by
-
Maternal Iron Deficiency Modulates Placental Transcriptome and Proteome in Mid-Gestation of Mouse Pregnancy.J Nutr. 2021 May 11;151(5):1073-1083. doi: 10.1093/jn/nxab005. J Nutr. 2021. PMID: 33693820 Free PMC article.
-
GATA/Heme Multi-omics Reveals a Trace Metal-Dependent Cellular Differentiation Mechanism.Dev Cell. 2018 Sep 10;46(5):581-594.e4. doi: 10.1016/j.devcel.2018.07.022. Epub 2018 Aug 16. Dev Cell. 2018. PMID: 30122630 Free PMC article.
-
The Functions of ZIP8, ZIP14, and ZnT10 in the Regulation of Systemic Manganese Homeostasis.Int J Mol Sci. 2020 May 7;21(9):3304. doi: 10.3390/ijms21093304. Int J Mol Sci. 2020. PMID: 32392784 Free PMC article. Review.
-
A blood pressure-associated variant of the SLC39A8 gene influences cellular cadmium accumulation and toxicity.Hum Mol Genet. 2016 Sep 15;25(18):4117-4126. doi: 10.1093/hmg/ddw236. Epub 2016 Jul 27. Hum Mol Genet. 2016. PMID: 27466201 Free PMC article.
-
Molecular and pathophysiological aspects of metal ion uptake by the zinc transporter ZIP8 (SLC39A8).Toxicol Res (Camb). 2016 Feb 18;5(4):987-1002. doi: 10.1039/c5tx00424a. eCollection 2016 Jul 1. Toxicol Res (Camb). 2016. PMID: 30090406 Free PMC article. Review.
References
-
- Eide DJ. The SLC39 family of metal ion transporters. Pflügers Archiv. 2004;447:796–800. - PubMed
-
- Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, et al. The acrodermatitis enteropathica gene Slc39a4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003;278:33474–33481. - PubMed
-
- Schmitt S, Kury S, Giraud M, Dreno B, Kharfi M, et al. An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum Mutat. 2009;30:926–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

