T regulatory cells control susceptibility to invasive pneumococcal pneumonia in mice
- PMID: 22563306
- PMCID: PMC3334885
- DOI: 10.1371/journal.ppat.1002660
T regulatory cells control susceptibility to invasive pneumococcal pneumonia in mice
Abstract
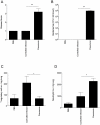
Streptococcus pneumoniae is an important human pathogen responsible for a spectrum of diseases including pneumonia. Immunological and pro-inflammatory processes induced in the lung during pneumococcal infection are well documented, but little is known about the role played by immunoregulatory cells and cytokines in the control of such responses. We demonstrate considerable differences in the immunomodulatory cytokine transforming growth factor (TGF)-β between the pneumococcal pneumonia resistant BALB/c and susceptible CBA/Ca mouse strains. Immunohistochemistry and flow cytometry reveal higher levels of TGF-β protein in BALB/c lungs during pneumococcal pneumonia that correlates with a rapid rise in lung Foxp3(+)Helios(+) T regulatory cells. These cells have protective functions during pneumococcal pneumonia, because blocking their induction with an inhibitor of TGF-β impairs BALB/c resistance to infection and aids bacterial dissemination from lungs. Conversely, adoptive transfer of T regulatory cells to CBA/Ca mice, prior to infection, prolongs survival and decreases bacterial dissemination from lungs to blood. Importantly, strong T regulatory cell responses also correlate with disease-resistance in outbred MF1 mice, confirming the importance of immunoregulatory cells in controlling protective responses to the pneumococcus. This study provides exciting new evidence for the importance of immunomodulation during pulmonary pneumococcal infection and suggests that TGF-β signalling is a potential target for immunotherapy or drug design.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

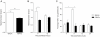
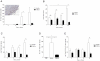



Similar articles
-
Protective Regulatory T Cell Immune Response Induced by Intranasal Immunization With the Live-Attenuated Pneumococcal Vaccine SPY1 via the Transforming Growth Factor-β1-Smad2/3 Pathway.Front Immunol. 2018 Aug 2;9:1754. doi: 10.3389/fimmu.2018.01754. eCollection 2018. Front Immunol. 2018. PMID: 30116243 Free PMC article.
-
Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection.Infect Immun. 2002 Mar;70(3):1547-57. doi: 10.1128/IAI.70.3.1547-1557.2002. Infect Immun. 2002. PMID: 11854243 Free PMC article.
-
Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway.J Exp Med. 2010 Oct 25;207(11):2331-41. doi: 10.1084/jem.20101074. Epub 2010 Sep 27. J Exp Med. 2010. PMID: 20876311 Free PMC article.
-
Of mice and men: innate immunity in pneumococcal pneumonia.Int J Antimicrob Agents. 2010 Feb;35(2):107-13. doi: 10.1016/j.ijantimicag.2009.10.002. Epub 2009 Dec 14. Int J Antimicrob Agents. 2010. PMID: 20005681 Review.
-
Immunogenicity differences of a 15-valent pneumococcal polysaccharide conjugate vaccine (PCV15) based on vaccine dose, route of immunization and mouse strain.Vaccine. 2017 Feb 7;35(6):865-872. doi: 10.1016/j.vaccine.2016.12.055. Epub 2017 Jan 10. Vaccine. 2017. PMID: 28087148 Review.
Cited by
-
Persisting high prevalence of pneumococcal carriage among HIV-infected adults receiving antiretroviral therapy in Malawi: a cohort study.AIDS. 2015 Sep 10;29(14):1837-44. doi: 10.1097/QAD.0000000000000755. AIDS. 2015. PMID: 26218599 Free PMC article.
-
Mechanisms of Naturally Acquired Immunity to Streptococcus pneumoniae.Front Immunol. 2019 Mar 1;10:358. doi: 10.3389/fimmu.2019.00358. eCollection 2019. Front Immunol. 2019. PMID: 30881363 Free PMC article. Review.
-
Reduced inflammation and cytokine production in NKLAM deficient mice during Streptococcus pneumoniae infection.PLoS One. 2018 Mar 8;13(3):e0194202. doi: 10.1371/journal.pone.0194202. eCollection 2018. PLoS One. 2018. PMID: 29518136 Free PMC article.
-
PM2.5 Causes Increased Bacterial Invasion by Affecting HBD1 Expression in the Lung.J Immunol Res. 2024 Jan 27;2024:6622950. doi: 10.1155/2024/6622950. eCollection 2024. J Immunol Res. 2024. PMID: 38314088 Free PMC article.
-
Diminished Pneumococcal-Specific CD4+ T-Cell Response is Associated With Increased Regulatory T Cells at Older Age.Front Aging. 2021 Nov 3;2:746295. doi: 10.3389/fragi.2021.746295. eCollection 2021. Front Aging. 2021. PMID: 35822055 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

