Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function
- PMID: 22556255
- PMCID: PMC3715993
- DOI: 10.1126/science.1216283
Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function
Abstract
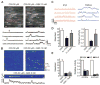
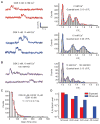
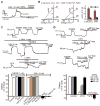
Major features of the transcellular signaling mechanism responsible for endothelium-dependent regulation of vascular smooth muscle tone are unresolved. We identified local calcium (Ca(2+)) signals ("sparklets") in the vascular endothelium of resistance arteries that represent Ca(2+) influx through single TRPV4 cation channels. Gating of individual TRPV4 channels within a four-channel cluster was cooperative, with activation of as few as three channels per cell causing maximal dilation through activation of endothelial cell intermediate (IK)- and small (SK)-conductance, Ca(2+)-sensitive potassium (K(+)) channels. Endothelial-dependent muscarinic receptor signaling also acted largely through TRPV4 sparklet-mediated stimulation of IK and SK channels to promote vasodilation. These results support the concept that Ca(2+) influx through single TRPV4 channels is leveraged by the amplifier effect of cooperative channel gating and the high Ca(2+) sensitivity of IK and SK channels to cause vasodilation.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

Comment in
-
Cell biology. Superresolution subspace signaling.Science. 2012 May 4;336(6081):546-7. doi: 10.1126/science.1222540. Science. 2012. PMID: 22556238 Free PMC article. No abstract available.
Similar articles
-
TRPV4 and the regulation of vascular tone.J Cardiovasc Pharmacol. 2013 Feb;61(2):113-9. doi: 10.1097/FJC.0b013e318279ba42. J Cardiovasc Pharmacol. 2013. PMID: 23107877 Free PMC article. Review.
-
Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels.Am J Physiol Heart Circ Physiol. 2016 Dec 1;311(6):H1437-H1444. doi: 10.1152/ajpheart.00465.2016. Epub 2016 Oct 7. Am J Physiol Heart Circ Physiol. 2016. PMID: 27765747 Free PMC article.
-
Physiological levels of fluid shear stress modulate vascular function through TRPV4 sparklets.Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(9):1268-1277. doi: 10.3724/abbs.2022118. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36082933 Free PMC article.
-
Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators.J Physiol. 2016 Jun 15;594(12):3271-85. doi: 10.1113/JP271652. Epub 2016 Mar 4. J Physiol. 2016. PMID: 26840527 Free PMC article.
-
Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels.J Cardiovasc Pharmacol. 2011 Feb;57(2):148-53. doi: 10.1097/FJC.0b013e3181f580d9. J Cardiovasc Pharmacol. 2011. PMID: 20729757 Review.
Cited by
-
Traumatic brain injury disrupts cerebrovascular tone through endothelial inducible nitric oxide synthase expression and nitric oxide gain of function.J Am Heart Assoc. 2014 Dec;3(6):e001474. doi: 10.1161/JAHA.114.001474. J Am Heart Assoc. 2014. PMID: 25527626 Free PMC article.
-
The role of cGMP/cGKI signalling and Trpc channels in regulation of vascular tone.Cardiovasc Res. 2013 Nov 1;100(2):280-7. doi: 10.1093/cvr/cvt176. Epub 2013 Jul 4. Cardiovasc Res. 2013. PMID: 23832809 Free PMC article.
-
TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension.Am J Physiol Cell Physiol. 2013 Oct 1;305(7):C704-15. doi: 10.1152/ajpcell.00099.2013. Epub 2013 Jun 5. Am J Physiol Cell Physiol. 2013. PMID: 23739180 Free PMC article.
-
TRPV4 and the regulation of vascular tone.J Cardiovasc Pharmacol. 2013 Feb;61(2):113-9. doi: 10.1097/FJC.0b013e318279ba42. J Cardiovasc Pharmacol. 2013. PMID: 23107877 Free PMC article. Review.
-
Endothelial Cell Calcium Influx Mediates Trauma-induced Endothelial Permeability.Ann Surg. 2023 Dec 11:10.1097/SLA.0000000000006164. doi: 10.1097/SLA.0000000000006164. Online ahead of print. Ann Surg. 2023. PMID: 38073572
References
-
- Saliez J, et al. Circulation. 2008;117:1065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM086736/GM/NIGMS NIH HHS/United States
- 2-P20-RR-016435-06/RR/NCRR NIH HHS/United States
- P01 HL095488/HL/NHLBI NIH HHS/United States
- P20 RR016435/RR/NCRR NIH HHS/United States
- R01 GM086736/GM/NIGMS NIH HHS/United States
- R01HL098243/HL/NHLBI NIH HHS/United States
- R37 DK053832/DK/NIDDK NIH HHS/United States
- HL044455/HL/NHLBI NIH HHS/United States
- R01 HL098243/HL/NHLBI NIH HHS/United States
- 1P01HL095488/HL/NHLBI NIH HHS/United States
- R37DK053832/DK/NIDDK NIH HHS/United States
- R01 HL044455/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

