The KSHV viral IL-6 homolog is sufficient to induce blood to lymphatic endothelial cell differentiation
- PMID: 22521915
- PMCID: PMC3509939
- DOI: 10.1016/j.virol.2012.03.013
The KSHV viral IL-6 homolog is sufficient to induce blood to lymphatic endothelial cell differentiation
Abstract
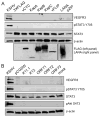
The predominant tumor cell of Kaposi's Sarcoma (KS) is the spindle cell, a cell of endothelial origin that expresses markers of lymphatic endothelium. In culture, Kaposi's Sarcoma-associated herpesvirus (KSHV) infection of blood endothelial cells drives expression of lymphatic endothelial cell specific markers, in a process that requires activation of the gp130 receptor and the JAK2/STAT3 and PI3K/AKT signaling pathways. While expression of each of the KSHV major latent genes in endothelial cells failed to increase expression of lymphatic markers, the viral homolog of human IL-6 (vIL-6) was sufficient for induction and requires the JAK2/STAT3 and PI3K/AKT pathways. Therefore, activation of gp130 and downstream signaling by vIL-6 is sufficient to drive blood to lymphatic endothelial cell differentiation. While sufficient, vIL-6 is not necessary for lymphatic reprogramming in the context of viral infection. This indicates that multiple viral genes are involved and suggests a central importance of this pathway to KSHV pathogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
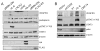
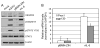



Similar articles
-
Activation of Akt through gp130 receptor signaling is required for Kaposi's sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells.J Virol. 2008 Sep;82(17):8771-9. doi: 10.1128/JVI.00766-08. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579585 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Viral Interleukin-6 Signaling Upregulates Integrin β3 Levels and Is Dependent on STAT3.J Virol. 2020 Feb 14;94(5):e01384-19. doi: 10.1128/JVI.01384-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801855 Free PMC article.
-
Persistent activation of STAT3 by latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells.J Virol. 2007 Mar;81(5):2449-58. doi: 10.1128/JVI.01769-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151100 Free PMC article.
-
Viral interleukin-6: role in Kaposi's sarcoma-associated herpesvirus: associated malignancies.J Interferon Cytokine Res. 2011 Nov;31(11):791-801. doi: 10.1089/jir.2011.0043. Epub 2011 Jul 18. J Interferon Cytokine Res. 2011. PMID: 21767154 Free PMC article. Review.
-
Kaposi's sarcoma herpesvirus-induced endothelial cell reprogramming supports viral persistence and contributes to Kaposi's sarcoma tumorigenesis.Curr Opin Virol. 2017 Oct;26:156-162. doi: 10.1016/j.coviro.2017.09.002. Epub 2017 Oct 12. Curr Opin Virol. 2017. PMID: 29031103 Review.
Cited by
-
The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside.Virology. 2015 May;479-480:568-77. doi: 10.1016/j.virol.2015.02.040. Epub 2015 Mar 20. Virology. 2015. PMID: 25798530 Free PMC article. Review.
-
Kaposi sarcoma herpesvirus pathogenesis.Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160275. doi: 10.1098/rstb.2016.0275. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28893942 Free PMC article. Review.
-
KSHV Induction of Angiogenic and Lymphangiogenic Phenotypes.Front Microbiol. 2012 Mar 30;3:102. doi: 10.3389/fmicb.2012.00102. eCollection 2012. Front Microbiol. 2012. PMID: 22479258 Free PMC article.
-
Tocilizumab monotherapy in a patient with rheumatoid arthritis and iatrogenic Kaposi sarcoma.Clin Drug Investig. 2014 Feb;34(2):159-61. doi: 10.1007/s40261-013-0159-9. Clin Drug Investig. 2014. PMID: 24307431
-
Modulation of Angiogenic Processes by the Human Gammaherpesviruses, Epstein-Barr Virus and Kaposi's Sarcoma-Associated Herpesvirus.Front Microbiol. 2019 Jul 12;10:1544. doi: 10.3389/fmicb.2019.01544. eCollection 2019. Front Microbiol. 2019. PMID: 31354653 Free PMC article. Review.
References
-
- Aluigi MG, Albini A, Carlone S, Repetto L, De Marchi R, Icardi A, Moro M, Noonan D, Benelli R. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi’s sarcoma. Res Virol. 1996;147:267–275. - PubMed
-
- Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027–1038. - PubMed
-
- Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, Moore PS, Tosato G. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93:4034–4043. - PubMed
-
- Boshoff C, Schulz TF, Kennedy MM, Graham AK, Fisher C, Thomas A, McGee JO, Weiss RA, O’Leary JJ. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

