Suppression of antigen-specific T cell responses by the Kaposi's sarcoma-associated herpesvirus viral OX2 protein and its cellular orthologue, CD200
- PMID: 22491458
- PMCID: PMC3372194
- DOI: 10.1128/JVI.07168-11
Suppression of antigen-specific T cell responses by the Kaposi's sarcoma-associated herpesvirus viral OX2 protein and its cellular orthologue, CD200
Abstract
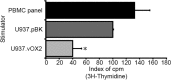
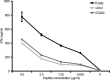
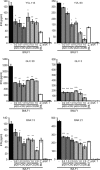
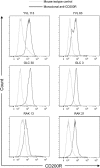
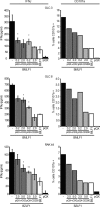
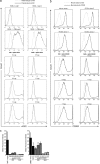
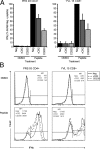
Regulating appropriate activation of the immune response in the healthy host despite continual immune surveillance dictates that immune responses must be either self-limiting and therefore negatively regulated following their activation or prevented from developing inappropriately. In the case of antigen-specific T cells, their response is attenuated by several mechanisms, including ligation of CTLA-4 and PD-1. Through the study of the viral OX2 (vOX2) immunoregulator encoded by Kaposi's sarcoma-associated herpesvirus (KSHV), we have identified a T cell-attenuating role both for this protein and for CD200, a cellular orthologue of the viral vOX2 protein. In vitro, antigen-presenting cells (APC) expressing either native vOX2 or CD200 suppressed two functions of cognate antigen-specific T cell clones: gamma interferon (IFN-γ) production and mobilization of CD107a, a cytolytic granule component and measure of target cell killing ability. Mechanistically, vOX2 and CD200 expression on APC suppressed the phosphorylation of ERK1/2 mitogen-activated protein kinase in responding T cells. These data provide the first evidence for a role of both KSHV vOX2 and cellular CD200 in the negative regulation of antigen-specific T cell responses. They suggest that KSHV has evolved to harness the host CD200-based mechanism of attenuation of T cell responses to facilitate virus persistence and dissemination within the infected individual. Moreover, our studies define a new paradigm in immune modulation by viruses: the provision of a negative costimulatory signal to T cells by a virus-encoded orthologue of CD200.
Figures


Similar articles
-
A new insight into viral proteins as Immunomodulatory therapeutic agents: KSHV vOX2 a homolog of human CD200 as a potent anti-inflammatory protein.Iran J Basic Med Sci. 2016 Jan;19(1):2-13. Iran J Basic Med Sci. 2016. PMID: 27096058 Free PMC article. Review.
-
vOX2 glycoprotein of human herpesvirus 8 modulates human primary macrophages activity.J Cell Physiol. 2009 Jun;219(3):698-706. doi: 10.1002/jcp.21722. J Cell Physiol. 2009. PMID: 19229882
-
Kaposi's sarcoma-associated herpesvirus inhibits expression and function of endothelial cell major histocompatibility complex class II via suppressor of cytokine signaling 3.J Virol. 2012 Jul;86(13):7158-66. doi: 10.1128/JVI.06908-11. Epub 2012 Apr 24. J Virol. 2012. PMID: 22532676 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus OX2 glycoprotein activates myeloid-lineage cells to induce inflammatory cytokine production.J Virol. 2002 May;76(10):4688-98. doi: 10.1128/jvi.76.10.4688-4698.2002. J Virol. 2002. PMID: 11967286 Free PMC article.
-
Immunological and inflammatory features of Kaposi's sarcoma and other Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-associated neoplasias.AIDS Rev. 2010 Jan-Mar;12(1):40-51. AIDS Rev. 2010. PMID: 20216909 Review.
Cited by
-
Rabbit CD200R binds host CD200 but not CD200-like proteins from poxviruses.Virology. 2016 Jan 15;488:1-8. doi: 10.1016/j.virol.2015.10.026. Epub 2015 Nov 17. Virology. 2016. PMID: 26590792 Free PMC article.
-
Professional antigen presenting cells in human herpesvirus 8 infection.Front Immunol. 2013 Jan 21;3:427. doi: 10.3389/fimmu.2012.00427. eCollection 2012. Front Immunol. 2013. PMID: 23346088 Free PMC article.
-
KSHV: Immune Modulation and Immunotherapy.Front Immunol. 2020 Feb 7;10:3084. doi: 10.3389/fimmu.2019.03084. eCollection 2019. Front Immunol. 2020. PMID: 32117196 Free PMC article. Review.
-
Interplay between Kaposi's sarcoma-associated herpesvirus and the innate immune system.Cytokine Growth Factor Rev. 2014 Oct;25(5):597-609. doi: 10.1016/j.cytogfr.2014.06.001. Epub 2014 Jun 21. Cytokine Growth Factor Rev. 2014. PMID: 25037686 Free PMC article. Review.
-
Cancel cancer: The immunotherapeutic potential of CD200/CD200R blockade.Front Oncol. 2023 Jan 23;13:1088038. doi: 10.3389/fonc.2023.1088038. eCollection 2023. Front Oncol. 2023. PMID: 36756156 Free PMC article. Review.
References
-
- Alberola-Ila J, Hernandez-Hoyos G. 2003. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 191:79–96 - PubMed
-
- Alegre ML, Frauwirth KA, Thompson CB. 2001. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 1:220–228 - PubMed
-
- Aresté C, Blackbourn DJ. 2009. Modulation of the immune system by Kaposi's sarcoma-associated herpesvirus. Trends Microbiol. 17:119–129 - PubMed
-
- Au-Yeung BB, et al. 2009. The structure, regulation, and function of ZAP-70. Immunol. Rev. 228:41–57 - PubMed
-
- Blackbourn DJ, et al. 1997. CD8+ cells from HIV-2-infected baboons control HIV replication. AIDS 11:737–746 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

