Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma
- PMID: 22454421
- PMCID: PMC3646316
- DOI: 10.1200/JCO.2011.38.0410
Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma
Abstract
Purpose: Brentuximab vedotin is an antibody-drug conjugate (ADC) that selectively delivers monomethyl auristatin E, an antimicrotubule agent, into CD30-expressing cells. In phase I studies, brentuximab vedotin demonstrated significant activity with a favorable safety profile in patients with relapsed or refractory CD30-positive lymphomas.
Patients and methods: In this multinational, open-label, phase II study, the efficacy and safety of brentuximab vedotin were evaluated in patients with relapsed or refractory Hodgkin's lymphoma (HL) after autologous stem-cell transplantation (auto-SCT). Patients had histologically documented CD30-positive HL by central pathology review. A total of 102 patients were treated with brentuximab vedotin 1.8 mg/kg by intravenous infusion every 3 weeks. In the absence of disease progression or prohibitive toxicity, patients received a maximum of 16 cycles. The primary end point was the overall objective response rate (ORR) determined by an independent radiology review facility.
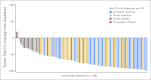
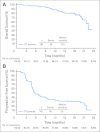
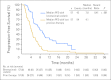
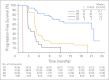
Results: The ORR was 75% with complete remission (CR) in 34% of patients. The median progression-free survival time for all patients was 5.6 months, and the median duration of response for those in CR was 20.5 months. After a median observation time of more than 1.5 years, 31 patients were alive and free of documented progressive disease. The most common treatment-related adverse events were peripheral sensory neuropathy, nausea, fatigue, neutropenia, and diarrhea.
Conclusion: The ADC brentuximab vedotin was associated with manageable toxicity and induced objective responses in 75% of patients with relapsed or refractory HL after auto-SCT. Durable CRs approaching 2 years were observed, supporting study in earlier lines of therapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Brentuximab vedotin and panobinostat: new drugs for Hodgkin's lymphoma--can they make one of medical oncology's chemotherapy success stories more successful?J Clin Oncol. 2012 Jun 20;30(18):2171-2. doi: 10.1200/JCO.2011.39.6416. Epub 2012 Apr 30. J Clin Oncol. 2012. PMID: 22547611 No abstract available.
-
Advances in LLM: Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma.Clin Adv Hematol Oncol. 2012 Jul;10(7):468-71. Clin Adv Hematol Oncol. 2012. PMID: 22895289 No abstract available.
Similar articles
-
Phase I / II study of brentuximab vedotin in Japanese patients with relapsed or refractory CD30-positive Hodgkin's lymphoma or systemic anaplastic large-cell lymphoma.Cancer Sci. 2014 Jul;105(7):840-6. doi: 10.1111/cas.12435. Epub 2014 Jul 1. Cancer Sci. 2014. PMID: 24814862 Free PMC article. Clinical Trial.
-
The European Medicines Agency Review of Brentuximab Vedotin (Adcetris) for the Treatment of Adult Patients With Relapsed or Refractory CD30+ Hodgkin Lymphoma or Systemic Anaplastic Large Cell Lymphoma: Summary of the Scientific Assessment of the Committee for Medicinal Products for Human Use.Oncologist. 2016 Jan;21(1):102-9. doi: 10.1634/theoncologist.2015-0276. Epub 2015 Nov 30. Oncologist. 2016. PMID: 26621039 Free PMC article.
-
Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single-arm, phase 1-2 trial.Lancet Oncol. 2018 Feb;19(2):257-266. doi: 10.1016/S1470-2045(17)30912-9. Epub 2017 Dec 21. Lancet Oncol. 2018. PMID: 29276022 Free PMC article. Clinical Trial.
-
Brentuximab vedotin for relapsed or refractory Hodgkin's lymphoma.Drug Des Devel Ther. 2015 Mar 23;9:1729-33. doi: 10.2147/DDDT.S82007. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25848209 Free PMC article. Review.
-
Brentuximab vedotin: an anti-CD30 antibody-drug conjugate.Am J Health Syst Pharm. 2013 Apr 1;70(7):589-97. doi: 10.2146/ajhp110608. Am J Health Syst Pharm. 2013. PMID: 23515511 Review.
Cited by
-
Comparison of the efficiency of ABVD versus BEACOPP for Hodgkin lymphoma treatment: a meta-analysis.Int J Hematol. 2016 Oct;104(4):413-9. doi: 10.1007/s12185-016-2080-5. Epub 2016 Aug 16. Int J Hematol. 2016. PMID: 27531149 Review.
-
Age-Related Changes in Pediatric Physiology: Quantitative Analysis of Organ Weights and Blood Flows : Age-Related Changes in Pediatric Physiology.AAPS J. 2021 Mar 31;23(3):50. doi: 10.1208/s12248-021-00581-1. AAPS J. 2021. PMID: 33791883
-
Results of a Multicenter Phase II Trial of Brentuximab Vedotin as Second-Line Therapy before Autologous Transplantation in Relapsed/Refractory Hodgkin Lymphoma.Biol Blood Marrow Transplant. 2015 Dec;21(12):2136-2140. doi: 10.1016/j.bbmt.2015.07.018. Epub 2015 Jul 26. Biol Blood Marrow Transplant. 2015. PMID: 26211987 Free PMC article. Clinical Trial.
-
Mesothelin is a novel cell surface disease marker and potential therapeutic target in acute myeloid leukemia.Blood Adv. 2021 May 11;5(9):2350-2361. doi: 10.1182/bloodadvances.2021004424. Blood Adv. 2021. PMID: 33938941 Free PMC article.
-
Treatment of relapsed classical Hodgkin lymphoma in the brentuximab vedotin era.Hematology Am Soc Hematol Educ Program. 2014 Dec 5;2014(1):151-7. doi: 10.1182/asheducation-2014.1.151. Epub 2014 Nov 18. Hematology Am Soc Hematol Educ Program. 2014. PMID: 25696848 Free PMC article. Review.
References
-
- Connors JM. State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin Oncol. 2005;23:6400–6408. - PubMed
-
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348:2386–2395. [Erratum: N Engl J Med 353:744, 2005] - PubMed
-
- Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–633. - PubMed
-
- Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–1072. - PubMed
-
- Horning S, Fanale M, deVos S, et al. Defining a population of Hodgkin lymphoma patients for novel therapeutics: An international effort. Ann Oncol. 2008;19:118. abstr.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

