IL-33 independently induces eosinophilic pericarditis and cardiac dilation: ST2 improves cardiac function
- PMID: 22454393
- PMCID: PMC3874395
- DOI: 10.1161/CIRCHEARTFAILURE.111.963769
IL-33 independently induces eosinophilic pericarditis and cardiac dilation: ST2 improves cardiac function
Abstract
Background: IL-33 through its receptor ST2 protects the heart from myocardial infarct and hypertrophy in animal models but, paradoxically, increases autoimmune disease. In this study, we examined the effect of IL-33 or ST2 administration on autoimmune heart disease.
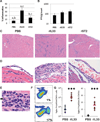
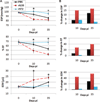
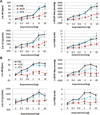
Methods and results: We used pressure-volume relationships and isoproterenol challenge to assess the effect of recombinant (r) IL-33 or rST2 (eg, soluble ST2) administration on the development of autoimmune coxsackievirus B3 myocarditis and dilated cardiomyopathy in male BALB/c mice. The rIL-33 treatment significantly increased acute perimyocarditis (P=0.006) and eosinophilia (P=1.3×10(-5)), impaired cardiac function (maximum ventricular power, P=0.0002), and increased ventricular dilation (end-diastolic volume, P=0.01). The rST2 treatment prevented eosinophilia and improved heart function compared with rIL-33 treatment (ejection fraction, P=0.009). Neither treatment altered viral replication. The rIL-33 treatment increased IL-4, IL-33, IL-1β, and IL-6 levels in the heart during acute myocarditis. To determine whether IL-33 altered cardiac function on its own, we administered rIL-33 to undiseased mice and found that rIL-33 induced eosinophilic pericarditis and adversely affected heart function. We used cytokine knockout mice to determine that this effect was due to IL-33-mediated signaling but not to IL-1β or IL-6.
Conclusions: We show for the first time to our knowledge that IL-33 induces eosinophilic pericarditis, whereas soluble ST2 prevents eosinophilia and improves systolic function, and that IL-33 independently adversely affects heart function through the IL-33 receptor.
Figures


Similar articles
-
Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling.Circ Heart Fail. 2009 Nov;2(6):684-91. doi: 10.1161/CIRCHEARTFAILURE.109.873240. Epub 2009 Sep 22. Circ Heart Fail. 2009. PMID: 19919994
-
Testosterone and interleukin-1β increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n.Am J Physiol Heart Circ Physiol. 2012 Apr 15;302(8):H1726-36. doi: 10.1152/ajpheart.00783.2011. Epub 2012 Feb 10. Am J Physiol Heart Circ Physiol. 2012. PMID: 22328081 Free PMC article.
-
IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system.J Clin Invest. 2007 Jun;117(6):1538-49. doi: 10.1172/JCI30634. Epub 2007 May 10. J Clin Invest. 2007. PMID: 17492053 Free PMC article.
-
Pathogenesis of myocarditis and dilated cardiomyopathy.Adv Immunol. 2008;99:95-114. doi: 10.1016/S0065-2776(08)00604-4. Adv Immunol. 2008. PMID: 19117533 Review.
-
Crucial and diverse role of the interleukin-33/ST2 axis in infectious diseases.Infect Immun. 2015 May;83(5):1738-48. doi: 10.1128/IAI.02908-14. Epub 2015 Feb 23. Infect Immun. 2015. PMID: 25712928 Free PMC article. Review.
Cited by
-
Sex and age differences in sST2 in cardiovascular disease.Front Cardiovasc Med. 2023 Jan 18;9:1073814. doi: 10.3389/fcvm.2022.1073814. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36741845 Free PMC article.
-
Increased Interleukin 18-Dependent Immune Responses Are Associated With Myopericarditis After COVID-19 mRNA Vaccination.Front Immunol. 2022 Feb 18;13:851620. doi: 10.3389/fimmu.2022.851620. eCollection 2022. Front Immunol. 2022. PMID: 35251049 Free PMC article.
-
Progress of Research into the Interleukin-1 Family in Cardiovascular Disease.J Inflamm Res. 2022 Dec 13;15:6683-6694. doi: 10.2147/JIR.S390915. eCollection 2022. J Inflamm Res. 2022. PMID: 36536642 Free PMC article. Review.
-
Sex and gender differences in myocarditis and dilated cardiomyopathy: An update.Front Cardiovasc Med. 2023 Mar 2;10:1129348. doi: 10.3389/fcvm.2023.1129348. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36937911 Free PMC article. Review.
-
Cardiomyocyte-specific transforming growth factor β suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction.Circ Res. 2014 Apr 11;114(8):1246-57. doi: 10.1161/CIRCRESAHA.114.302653. Epub 2014 Feb 26. Circ Res. 2014. PMID: 24573206 Free PMC article.
References
-
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics- 2011 update. Circulation. 2011;123:e18–e209. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

