Phase II study of bevacizumab in patients with HIV-associated Kaposi's sarcoma receiving antiretroviral therapy
- PMID: 22430271
- PMCID: PMC3383119
- DOI: 10.1200/JCO.2011.39.6853
Phase II study of bevacizumab in patients with HIV-associated Kaposi's sarcoma receiving antiretroviral therapy
Abstract
Purpose: Alternatives to cytotoxic agents are desirable for patients with HIV-associated Kaposi's sarcoma (KS). Vascular endothelial growth factor-A (VEGF-A) contributes to KS pathogenesis. We evaluated the humanized anti-VEGF-A monoclonal antibody, bevacizumab, in patients with HIV-KS.
Patients and methods: Patients with HIV-KS who either experienced progression while receiving highly active antiretroviral therapy (HAART) for at least 1 month or did not regress despite HAART for at least 4 months were administered bevacizumab 15 mg/kg intravenously on days 1 and 8 and then every 3 weeks. The primary objective was assessment of antitumor activity using modified AIDS Clinical Trial Group (ACTG) criteria for HIV-KS. HIV-uninfected patients were also eligible and observed separately.
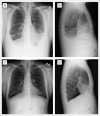
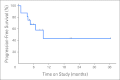
Results: Seventeen HIV-infected patients were enrolled. Fourteen patients had been receiving effective HAART for at least 6 months (median, 1 year). Thirteen patients had advanced disease (ACTG T(1)), 13 patients had received prior chemotherapy for KS, and seven patients had CD4 count less than 200 cells/μL. Median number of cycles was 10 (range, 1 to 37 cycles); median follow-up was 8.3 months (range, 3 to 36 months). Of 16 assessable patients, best tumor responses observed were complete response (CR) in three patients (19%), partial response (PR) in two patients (12%), stable disease in nine patients (56%), and progressive disease in two patients (12%). Overall response rate (CR + PR) was 31% (95% CI, 11% to 58.7%). Four of five responders had received prior chemotherapy for KS. Over 202 cycles, grade 3 to 4 adverse events at least possibly attributed to therapy included hypertension (n = 7), neutropenia (n = 5), cellulitis (n = 3), and headache (n = 2).
Conclusion: Bevacizumab is tolerated in patients with HIV-KS and has activity in a subset of patients.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Intralesional bevacizumab in patients with human immunodeficiency virus-associated Kaposi's sarcoma in the upper airway.Laryngoscope. 2015 Apr;125(4):E132-7. doi: 10.1002/lary.24988. Epub 2014 Oct 27. Laryngoscope. 2015. PMID: 25345840 Clinical Trial.
-
Phase II Trial of Lenalidomide in HIV-Infected Patients with Previously Treated Kaposi's Sarcoma: Results of the ANRS 154 Lenakap Trial.AIDS Res Hum Retroviruses. 2017 Jan;33(1):1-10. doi: 10.1089/AID.2016.0069. Epub 2016 Sep 7. AIDS Res Hum Retroviruses. 2017. PMID: 27405442 Clinical Trial.
-
Pomalidomide for Symptomatic Kaposi's Sarcoma in People With and Without HIV Infection: A Phase I/II Study.J Clin Oncol. 2016 Dec;34(34):4125-4131. doi: 10.1200/JCO.2016.69.3812. Epub 2016 Oct 31. J Clin Oncol. 2016. PMID: 27863194 Free PMC article. Clinical Trial.
-
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults.Cochrane Database Syst Rev. 2014 Aug 13;8(8):CD003256. doi: 10.1002/14651858.CD003256.pub2. Cochrane Database Syst Rev. 2014. PMID: 25221796 Free PMC article. Review.
-
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults.Cochrane Database Syst Rev. 2014;(9):CD003256. doi: 10.1002/14651858.CD003256.pub2. Cochrane Database Syst Rev. 2014. PMID: 25313415 Review.
Cited by
-
Molecular Biology of KSHV in Relation to HIV/AIDS-Associated Oncogenesis.Cancer Treat Res. 2019;177:23-62. doi: 10.1007/978-3-030-03502-0_2. Cancer Treat Res. 2019. PMID: 30523620 Free PMC article.
-
Modulation of Angiogenic Processes by the Human Gammaherpesviruses, Epstein-Barr Virus and Kaposi's Sarcoma-Associated Herpesvirus.Front Microbiol. 2019 Jul 12;10:1544. doi: 10.3389/fmicb.2019.01544. eCollection 2019. Front Microbiol. 2019. PMID: 31354653 Free PMC article. Review.
-
Predictive Value of Serum Biomarkers for Response of Limited-Stage AIDS-Associated Kaposi Sarcoma to Antiretroviral Therapy With or Without Concomitant Chemotherapy in Resource-Limited Settings.J Acquir Immune Defic Syndr. 2023 Oct 1;94(2):165-173. doi: 10.1097/QAI.0000000000003236. J Acquir Immune Defic Syndr. 2023. PMID: 37368929 Free PMC article.
-
Kaposi sarcoma herpesvirus-associated cancers and related diseases.Curr Opin HIV AIDS. 2017 Jan;12(1):47-56. doi: 10.1097/COH.0000000000000330. Curr Opin HIV AIDS. 2017. PMID: 27662501 Free PMC article. Review.
-
Safety and effectiveness of gemcitabine for the treatment of classic Kaposi's sarcoma without visceral involvement.Ther Adv Med Oncol. 2022 Mar 26;14:17588359221086829. doi: 10.1177/17588359221086829. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 35356263 Free PMC article.
References
-
- Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Whitby D, Howard MR, Tenant-Flowers M, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet. 1995;346:799–802. - PubMed
-
- Moore PS, Kingsley LA, Holmberg SD, et al. Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS. 1996;10:175–180. - PubMed
-
- Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

