In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo
- PMID: 22411804
- PMCID: PMC3323997
- DOI: 10.1073/pnas.1110203109
In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo
Abstract
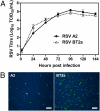
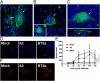
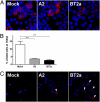
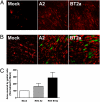
Respiratory syncytial virus (RSV) is the major viral cause of severe pulmonary disease in young infants worldwide. However, the mechanisms by which RSV causes disease in humans remain poorly understood. To help bridge this gap, we developed an ex vivo/in vitro model of RSV infection based on well-differentiated primary pediatric bronchial epithelial cells (WD-PBECs), the primary targets of RSV infection in vivo. Our RSV/WD-PBEC model demonstrated remarkable similarities to hallmarks of RSV infection in infant lungs. These hallmarks included restriction of infection to noncontiguous or small clumps of apical ciliated and occasional nonciliated epithelial cells, apoptosis and sloughing of apical epithelial cells, occasional syncytium formation, goblet cell hyperplasia/metaplasia, and mucus hypersecretion. RSV was shed exclusively from the apical surface at titers consistent with those in airway aspirates from hospitalized infants. Furthermore, secretion of proinflammatory chemokines such as CXCL10, CCL5, IL-6, and CXCL8 reflected those chemokines present in airway aspirates. Interestingly, a recent RSV clinical isolate induced more cytopathogenesis than the prototypic A2 strain. Our findings indicate that this RSV/WD-PBEC model provides an authentic surrogate for RSV infection of airway epithelium in vivo. As such, this model may provide insights into RSV pathogenesis in humans that ultimately lead to successful RSV vaccines or therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium.Am J Respir Crit Care Med. 2013 Oct 1;188(7):842-51. doi: 10.1164/rccm.201304-0750OC. Am J Respir Crit Care Med. 2013. PMID: 23952745
-
Induction and Antagonism of Antiviral Responses in Respiratory Syncytial Virus-Infected Pediatric Airway Epithelium.J Virol. 2015 Dec;89(24):12309-18. doi: 10.1128/JVI.02119-15. Epub 2015 Sep 30. J Virol. 2015. PMID: 26423940 Free PMC article.
-
Differential cytopathogenesis of respiratory syncytial virus prototypic and clinical isolates in primary pediatric bronchial epithelial cells.Virol J. 2011 Jan 27;8:43. doi: 10.1186/1743-422X-8-43. Virol J. 2011. PMID: 21272337 Free PMC article.
-
Respiratory syncytial virus interaction with human airway epithelium.Trends Microbiol. 2013 May;21(5):238-44. doi: 10.1016/j.tim.2013.02.004. Epub 2013 Mar 22. Trends Microbiol. 2013. PMID: 23523320 Review.
-
Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response.Microbes Infect. 2013 Mar;15(3):230-42. doi: 10.1016/j.micinf.2012.11.012. Epub 2012 Dec 12. Microbes Infect. 2013. PMID: 23246463 Review.
Cited by
-
3D engineered tissue models for studying human-specific infectious viral diseases.Bioact Mater. 2022 Sep 22;21:576-594. doi: 10.1016/j.bioactmat.2022.09.010. eCollection 2023 Mar. Bioact Mater. 2022. PMID: 36204281 Free PMC article. Review.
-
T-cell immunoglobulin and mucin domain 1 deficiency eliminates airway hyperreactivity triggered by the recognition of airway cell death.J Allergy Clin Immunol. 2013 Aug;132(2):414-25.e6. doi: 10.1016/j.jaci.2013.03.025. Epub 2013 May 11. J Allergy Clin Immunol. 2013. PMID: 23672783 Free PMC article.
-
Late therapeutic intervention with a respiratory syncytial virus L-protein polymerase inhibitor, PC786, on respiratory syncytial virus infection in human airway epithelium.Br J Pharmacol. 2018 Jun;175(12):2520-2534. doi: 10.1111/bph.14221. Epub 2018 May 2. Br J Pharmacol. 2018. PMID: 29579332 Free PMC article.
-
Commentary: Blood Eosinophilia Is Associated with Unfavorable Hospitalization Outcomes in Children with Bronchiolitis.Front Pediatr. 2016 Nov 21;4:123. doi: 10.3389/fped.2016.00123. eCollection 2016. Front Pediatr. 2016. PMID: 27965947 Free PMC article. No abstract available.
-
Primary differentiated respiratory epithelial cells respond to apical measles virus infection by shedding multinucleated giant cells.Proc Natl Acad Sci U S A. 2021 Mar 16;118(11):e2013264118. doi: 10.1073/pnas.2013264118. Proc Natl Acad Sci U S A. 2021. PMID: 33836570 Free PMC article.
References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. - PubMed
-
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. - PubMed
-
- Stein RT, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. - PubMed
-
- Sigurs N, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. - PubMed
-
- McNamara PS, Flanagan BF, Hart CA, Smyth RL. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2005;191:1225–1232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

