Kaposi's sarcoma herpesvirus upregulates Aurora A expression to promote p53 phosphorylation and ubiquitylation
- PMID: 22396649
- PMCID: PMC3291660
- DOI: 10.1371/journal.ppat.1002566
Kaposi's sarcoma herpesvirus upregulates Aurora A expression to promote p53 phosphorylation and ubiquitylation
Abstract
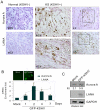
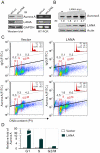
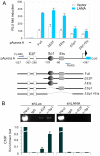
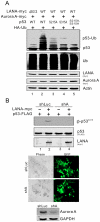
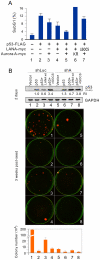
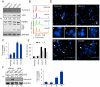
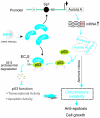
Aberrant expression of Aurora A kinase has been frequently implicated in many cancers and contributes to chromosome instability and phosphorylation-mediated ubiquitylation and degradation of p53 for tumorigenesis. Previous studies showed that p53 is degraded by Kaposi's sarcoma herpesvirus (KSHV) encoded latency-associated nuclear antigen (LANA) through its SOCS-box (suppressor of cytokine signaling, LANA(SOCS)) motif-mediated recruitment of the EC(5)S ubiquitin complex. Here we demonstrate that Aurora A transcriptional expression is upregulated by LANA and markedly elevated in both Kaposi's sarcoma tissue and human primary cells infected with KSHV. Moreover, reintroduction of Aurora A dramatically enhances the binding affinity of p53 with LANA and LANA(SOCS)-mediated ubiquitylation of p53 which requires phosphorylation on Ser215 and Ser315. Small hairpin RNA or a dominant negative mutant of Aurora A kinase efficiently disrupts LANA-induced p53 ubiquitylation and degradation, and leads to induction of p53 transcriptional and apoptotic activities. These studies provide new insights into the mechanisms by which LANA can upregulate expression of a cellular oncogene and simultaneously destabilize the activities of the p53 tumor suppressor in KSHV-associated human cancers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

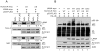
Similar articles
-
Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (KSHV) upregulates survivin expression in KSHV-Associated B-lymphoma cells and contributes to their proliferation.J Virol. 2009 Jul;83(14):7129-41. doi: 10.1128/JVI.00397-09. Epub 2009 May 13. J Virol. 2009. PMID: 19439469 Free PMC article.
-
EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors.PLoS Pathog. 2006 Oct;2(10):e116. doi: 10.1371/journal.ppat.0020116. PLoS Pathog. 2006. PMID: 17069461 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus LANA Modulates the Stability of the E3 Ubiquitin Ligase RLIM.J Virol. 2020 Feb 14;94(5):e01578-19. doi: 10.1128/JVI.01578-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801865 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-encoded LANA contributes to viral latent replication by activating phosphorylation of survivin.J Virol. 2014 Apr;88(8):4204-17. doi: 10.1128/JVI.03855-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478433 Free PMC article.
-
WHAT do viruses BET on?Front Biosci (Landmark Ed). 2010 Jan 1;15(2):537-49. doi: 10.2741/3632. Front Biosci (Landmark Ed). 2010. PMID: 20036832 Review.
Cited by
-
DNA-binding protects p53 from interactions with cofactors involved in transcription-independent functions.Nucleic Acids Res. 2016 Nov 2;44(19):9096-9109. doi: 10.1093/nar/gkw770. Epub 2016 Sep 6. Nucleic Acids Res. 2016. PMID: 27604871 Free PMC article.
-
Epstein - Barr virus transforming protein LMP-1 alters B cells gene expression by promoting accumulation of the oncoprotein ΔNp73α.PLoS Pathog. 2013 Mar;9(3):e1003186. doi: 10.1371/journal.ppat.1003186. Epub 2013 Mar 14. PLoS Pathog. 2013. PMID: 23516355 Free PMC article.
-
A Screen for Extracellular Signal-Regulated Kinase-Primed Glycogen Synthase Kinase 3 Substrates Identifies the p53 Inhibitor iASPP.J Virol. 2015 Sep;89(18):9232-41. doi: 10.1128/JVI.01072-15. Epub 2015 Jun 24. J Virol. 2015. PMID: 26109723 Free PMC article.
-
Constitutive Activation of Interleukin-13/STAT6 Contributes to Kaposi's Sarcoma-Associated Herpesvirus-Related Primary Effusion Lymphoma Cell Proliferation and Survival.J Virol. 2015 Oct;89(20):10416-26. doi: 10.1128/JVI.01525-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246572 Free PMC article.
-
Simulations of mutant p53 DNA binding domains reveal a novel druggable pocket.Nucleic Acids Res. 2019 Feb 28;47(4):1637-1652. doi: 10.1093/nar/gky1314. Nucleic Acids Res. 2019. PMID: 30649466 Free PMC article.
References
-
- Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95:1406–1412. - PubMed
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. - PubMed
-
- Godfrey A, Anderson J, Papanastasiou A, Takeuchi Y, Boshoff C. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood. 2005;105:2510–2518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R01CA138434-01A209/CA/NCI NIH HHS/United States
- 5R01CA091792-08/CA/NCI NIH HHS/United States
- R01 CA138434/CA/NCI NIH HHS/United States
- 1R01CA137894-01/CA/NCI NIH HHS/United States
- R01 CA091792/CA/NCI NIH HHS/United States
- R01 AI067037/AI/NIAID NIH HHS/United States
- 5R01CA108461-05/CA/NCI NIH HHS/United States
- 5R01AI067037-04/AI/NIAID NIH HHS/United States
- R01 DE017338/DE/NIDCR NIH HHS/United States
- 5R01DE017338-03/DE/NIDCR NIH HHS/United States
- R01 CA108461/CA/NCI NIH HHS/United States
- R01 CA137894/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

