Direct and efficient cellular transformation of primary rat mesenchymal precursor cells by KSHV
- PMID: 22293176
- PMCID: PMC3287217
- DOI: 10.1172/JCI58530
Direct and efficient cellular transformation of primary rat mesenchymal precursor cells by KSHV
Abstract
Infections by viruses are associated with approximately 12% of human cancer. Kaposi's sarcoma-associated herpesvirus (KSHV) is causally linked to several malignancies commonly found in AIDS patients. The mechanism of KSHV-induced oncogenesis remains elusive, due in part to the lack of an adequate experimental system for cellular transformation of primary cells. Here, we report efficient infection and cellular transformation of primary rat embryonic metanephric mesenchymal precursor cells (MM cells) by KSHV. Cellular transformation occurred at as early as day 4 after infection and in nearly all infected cells. Transformed cells expressed hallmark vascular endothelial, lymphatic endothelial, and mesenchymal markers and efficiently induced tumors in nude mice. KSHV established latent infection in MM cells, and lytic induction resulted in low levels of detectable infectious virions despite robust expression of lytic genes. Most KSHV-induced tumor cells were in a latent state, although a few showed heterogeneous expression of lytic genes. This efficient system for KSHV cellular transformation of primary cells might facilitate the study of growth deregulation mechanisms resulting from KSHV infections.
Figures
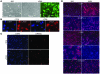
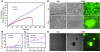
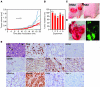
Similar articles
-
Kaposi's Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells.J Virol. 2020 Mar 31;94(8):e01692-19. doi: 10.1128/JVI.01692-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969437 Free PMC article.
-
Targeting Kaposi's Sarcoma-Associated Herpesvirus ORF21 Tyrosine Kinase and Viral Lytic Reactivation by Tyrosine Kinase Inhibitors Approved for Clinical Use.J Virol. 2020 Feb 14;94(5):e01791-19. doi: 10.1128/JVI.01791-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31826996 Free PMC article.
-
Screening of the Human Kinome Identifies MSK1/2-CREB1 as an Essential Pathway Mediating Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication during Primary Infection.J Virol. 2015 Sep;89(18):9262-80. doi: 10.1128/JVI.01098-15. Epub 2015 Jun 24. J Virol. 2015. PMID: 26109721 Free PMC article.
-
Kaposi's sarcoma herpesvirus-induced endothelial cell reprogramming supports viral persistence and contributes to Kaposi's sarcoma tumorigenesis.Curr Opin Virol. 2017 Oct;26:156-162. doi: 10.1016/j.coviro.2017.09.002. Epub 2017 Oct 12. Curr Opin Virol. 2017. PMID: 29031103 Review.
-
Viral latent proteins as targets for Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) induced lymphoma.Curr Drug Targets Infect Disord. 2003 Jun;3(2):129-35. doi: 10.2174/1568005033481150. Curr Drug Targets Infect Disord. 2003. PMID: 12769790 Review.
Cited by
-
Oncogenes and RNA splicing of human tumor viruses.Emerg Microbes Infect. 2014 Sep;3(9):e63. doi: 10.1038/emi.2014.62. Epub 2014 Sep 3. Emerg Microbes Infect. 2014. PMID: 26038756 Free PMC article. Review.
-
Methylation of KSHV vCyclin by PRMT5 contributes to cell cycle progression and cell proliferation.PLoS Pathog. 2024 Sep 10;20(9):e1012535. doi: 10.1371/journal.ppat.1012535. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39255317 Free PMC article.
-
Oncogenic Kaposi's Sarcoma-Associated Herpesvirus Upregulates Argininosuccinate Synthase 1, a Rate-Limiting Enzyme of the Citrulline-Nitric Oxide Cycle, To Activate the STAT3 Pathway and Promote Growth Transformation.J Virol. 2019 Feb 5;93(4):e01599-18. doi: 10.1128/JVI.01599-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463977 Free PMC article.
-
NDRG1 facilitates the replication and persistence of Kaposi's sarcoma-associated herpesvirus by interacting with the DNA polymerase clamp PCNA.PLoS Pathog. 2019 Feb 27;15(2):e1007628. doi: 10.1371/journal.ppat.1007628. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30811506 Free PMC article.
-
RNA epitranscriptomics: Regulation of infection of RNA and DNA viruses by N6 -methyladenosine (m6 A).Rev Med Virol. 2018 Jul;28(4):e1983. doi: 10.1002/rmv.1983. Epub 2018 Apr 26. Rev Med Virol. 2018. PMID: 29698584 Free PMC article. Review.
References
-
- Ciufo DM, et al. Spindle cell conversion by Kaposi’s sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J Virol. 2001;75(12):5614–5626. doi: 10.1128/JVI.75.12.5614-5626.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- CA132637/CA/NCI NIH HHS/United States
- R01 CA096512-09/CA/NCI NIH HHS/United States
- R01 CA124332-03/CA/NCI NIH HHS/United States
- R01 CA132637-04/CA/NCI NIH HHS/United States
- R01 CA096512-06/CA/NCI NIH HHS/United States
- R01 CA132637-02/CA/NCI NIH HHS/United States
- R01 CA124332-04/CA/NCI NIH HHS/United States
- CA096512/CA/NCI NIH HHS/United States
- R01 CA132637-02S1/CA/NCI NIH HHS/United States
- R01 CA124332-01A2/CA/NCI NIH HHS/United States
- R01 CA132637/CA/NCI NIH HHS/United States
- R01 CA124332-06/CA/NCI NIH HHS/United States
- CA124332/CA/NCI NIH HHS/United States
- R01 CA132637-01A1/CA/NCI NIH HHS/United States
- R01 CA096512-07/CA/NCI NIH HHS/United States
- R01 CA124332-02/CA/NCI NIH HHS/United States
- R01 CA132637-06/CA/NCI NIH HHS/United States
- R01 CA096512/CA/NCI NIH HHS/United States
- R01 CA132637-03S1/CA/NCI NIH HHS/United States
- R01 CA124332/CA/NCI NIH HHS/United States
- R01 CA132637-03/CA/NCI NIH HHS/United States
- R01 CA096512-08/CA/NCI NIH HHS/United States
- R01 CA132637-04S1/CA/NCI NIH HHS/United States
- R01 CA132637-05/CA/NCI NIH HHS/United States
- R01 CA124332-05/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

