Noncanonical NF-κB signaling regulates hematopoietic stem cell self-renewal and microenvironment interactions
- PMID: 22290873
- PMCID: PMC3602314
- DOI: 10.1002/stem.1050
Noncanonical NF-κB signaling regulates hematopoietic stem cell self-renewal and microenvironment interactions
Abstract
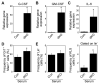
RelB and nuclear factor κB (NF-κB2) are the main effectors of NF-κB noncanonical signaling and play critical roles in many physiological processes. However, their role in hematopoietic stem/progenitor cell (HSPC) maintenance has not been characterized. To investigate this, we generated RelB/NF-κB2 double-knockout (dKO) mice and found that dKO HSPCs have profoundly impaired engraftment and self-renewal activity after transplantation into wild-type recipients. Transplantation of wild-type bone marrow cells into dKO mice to assess the role of the dKO microenvironment showed that wild-type HSPCs cycled more rapidly, were more abundant, and had developmental aberrancies: increased myeloid and decreased lymphoid lineages, similar to dKO HSPCs. Notably, when these wild-type cells were returned to normal hosts, these phenotypic changes were reversed, indicating a potent but transient phenotype conferred by the dKO microenvironment. However, dKO bone marrow stromal cell numbers were reduced, and bone-lining niche cells supported less HSPC expansion than controls. Furthermore, increased dKO HSPC proliferation was associated with impaired expression of niche adhesion molecules by bone-lining cells and increased inflammatory cytokine expression by bone marrow cells. Thus, RelB/NF-κB2 signaling positively and intrinsically regulates HSPC self-renewal and maintains stromal/osteoblastic niches and negatively and extrinsically regulates HSPC expansion and lineage commitment through the marrow microenvironment.
Copyright © 2012 AlphaMed Press.
Figures

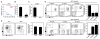

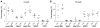

Similar articles
-
Three-dimensional co-culture of mesenchymal stromal cells and differentiated osteoblasts on human bio-derived bone scaffolds supports active multi-lineage hematopoiesis in vitro: Functional implication of the biomimetic HSC niche.Int J Mol Med. 2016 Oct;38(4):1141-51. doi: 10.3892/ijmm.2016.2712. Epub 2016 Aug 19. Int J Mol Med. 2016. PMID: 27571775 Free PMC article.
-
Constitutive Activation of NIK Impairs the Self-Renewal of Hematopoietic Stem/Progenitor Cells and Induces Bone Marrow Failure.Stem Cells. 2017 Mar;35(3):777-786. doi: 10.1002/stem.2523. Epub 2016 Nov 2. Stem Cells. 2017. PMID: 27733012 Free PMC article.
-
SWEF Proteins Distinctly Control Maintenance and Differentiation of Hematopoietic Stem Cells.PLoS One. 2016 Aug 25;11(8):e0161060. doi: 10.1371/journal.pone.0161060. eCollection 2016. PLoS One. 2016. PMID: 27561029 Free PMC article.
-
Advances in umbilical cord blood stem cell expansion and clinical translation.Exp Hematol. 2015 Jul;43(7):498-513. doi: 10.1016/j.exphem.2015.04.011. Epub 2015 May 10. Exp Hematol. 2015. PMID: 25970610 Review.
-
Neurological Regulation of the Bone Marrow Niche.Adv Exp Med Biol. 2020;1212:127-153. doi: 10.1007/5584_2019_398. Adv Exp Med Biol. 2020. PMID: 31342461 Review.
Cited by
-
A role for BMP-induced homeobox gene MIXL1 in acute myelogenous leukemia and identification of type I BMP receptor as a potential target for therapy.Oncotarget. 2014 Dec 30;5(24):12675-93. doi: 10.18632/oncotarget.2564. Oncotarget. 2014. PMID: 25544748 Free PMC article.
-
MicroRNA-21 maintains hematopoietic stem cell homeostasis through sustaining the NF-κB signaling pathway in mice.Haematologica. 2021 Feb 1;106(2):412-423. doi: 10.3324/haematol.2019.236927. Haematologica. 2021. PMID: 31974197 Free PMC article.
-
Leptin receptor, a surface marker for a subset of highly engrafting long-term functional hematopoietic stem cells.Leukemia. 2021 Jul;35(7):2064-2075. doi: 10.1038/s41375-020-01079-z. Epub 2020 Nov 7. Leukemia. 2021. PMID: 33159180 Free PMC article.
-
RNA m5C oxidation by TET2 regulates chromatin state and leukaemogenesis.Nature. 2024 Oct;634(8035):986-994. doi: 10.1038/s41586-024-07969-x. Epub 2024 Oct 2. Nature. 2024. PMID: 39358506 Free PMC article.
-
Constitutive Activation of NF-κB Pathway in Hematopoietic Stem Cells Causes Loss of Quiescence and Deregulated Transcription Factor Networks.Front Cell Dev Biol. 2018 Oct 30;6:143. doi: 10.3389/fcell.2018.00143. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30425986 Free PMC article.
References
-
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. - PubMed
-
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. - PubMed
-
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

