Serum amyloid A in uremic HDL promotes inflammation
- PMID: 22282592
- PMCID: PMC3338291
- DOI: 10.1681/ASN.2011070668
Serum amyloid A in uremic HDL promotes inflammation
Abstract
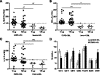
Uremia impairs the atheroprotective properties of HDL, but the mechanisms underlying why this occurs are unknown. Here, we observed that HDL isolated from healthy individuals inhibited the production of inflammatory cytokines by peripheral monocytes stimulated with a Toll-like receptor 2 agonist. In contrast, HDL isolated from the majority of patients with ESRD did not show this anti-inflammatory property; many HDL samples even promoted the production of inflammatory cytokines. To investigate this difference, we used shotgun proteomics to identify 49 HDL-associated proteins in a uremia-specific pattern. Proteins enriched in HDL from patients with ESRD (ESRD-HDL) included surfactant protein B (SP-B), apolipoprotein C-II, serum amyloid A (SAA), and α-1-microglobulin/bikunin precursor. In addition, we detected some ESRD-enriched proteins in earlier stages of CKD. We did not detect a difference in oxidation status between HDL isolated from uremic and healthy patients. Regarding function of these uremia-specific proteins, only SAA mimicked ESRD-HDL by promoting inflammatory cytokine production. Furthermore, SAA levels in ESRD-HDL inversely correlated with its anti-inflammatory potency. In conclusion, HDL has anti-inflammatory activities that are defective in uremic patients as a result of specific changes in its molecular composition. These data suggest a potential link between the high levels of inflammation and cardiovascular mortality in uremia.
Figures
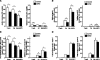




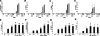
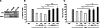
Similar articles
-
High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A.Cardiovasc Res. 2012 Apr 1;94(1):154-62. doi: 10.1093/cvr/cvs089. Epub 2012 Feb 10. Cardiovasc Res. 2012. PMID: 22328092
-
Restoration of renal function does not correct impairment of uremic HDL properties.J Am Soc Nephrol. 2015 Mar;26(3):565-75. doi: 10.1681/ASN.2013111219. Epub 2014 Jul 28. J Am Soc Nephrol. 2015. PMID: 25071090 Free PMC article.
-
Uremia alters HDL composition and function.J Am Soc Nephrol. 2011 Sep;22(9):1631-41. doi: 10.1681/ASN.2010111144. Epub 2011 Jul 29. J Am Soc Nephrol. 2011. PMID: 21804091 Free PMC article.
-
Molecular mechanisms underlying uremic toxin-related systemic disorders in chronic kidney disease: focused on β2-microglobulin-related amyloidosis and indoxyl sulfate-induced atherosclerosis-Oshima Award Address 2016.Clin Exp Nephrol. 2019 Feb;23(2):151-157. doi: 10.1007/s10157-018-1588-9. Epub 2018 Jun 5. Clin Exp Nephrol. 2019. PMID: 29869756 Free PMC article. Review.
-
[Changes in biological functions of high-density lipoprotein after abnormal modification].Sheng Li Xue Bao. 2017 Apr 25;69(2):225-234. Sheng Li Xue Bao. 2017. PMID: 28435982 Review. Chinese.
Cited by
-
The Endothelium Is Both a Target and a Barrier of HDL's Protective Functions.Cells. 2021 Apr 28;10(5):1041. doi: 10.3390/cells10051041. Cells. 2021. PMID: 33924941 Free PMC article. Review.
-
Subfractions of high-density lipoprotein (HDL) and dysfunctional HDL in chronic kidney disease patients.Arch Med Sci. 2016 Aug 1;12(4):844-9. doi: 10.5114/aoms.2016.60971. Epub 2016 Jul 1. Arch Med Sci. 2016. PMID: 27478466 Free PMC article.
-
Distinct Proteomic Signatures in 16 HDL (High-Density Lipoprotein) Subspecies.Arterioscler Thromb Vasc Biol. 2018 Dec;38(12):2827-2842. doi: 10.1161/ATVBAHA.118.311607. Arterioscler Thromb Vasc Biol. 2018. PMID: 30571168 Free PMC article.
-
Dysfunctional high-density lipoproteins in children with chronic kidney disease.Metabolism. 2015 Feb;64(2):263-73. doi: 10.1016/j.metabol.2014.10.020. Epub 2014 Oct 25. Metabolism. 2015. PMID: 25467845 Free PMC article.
-
Antipsoriatic treatment extends beyond the skin: recovering of high-density lipoprotein function.Exp Dermatol. 2014 Oct;23(10):701-4. doi: 10.1111/exd.12483. Epub 2014 Jul 31. Exp Dermatol. 2014. PMID: 24980461 Free PMC article. Review.
References
-
- Shoham DA, Vupputuri S, Kshirsagar AV: Chronic kidney disease and life course socioeconomic status: A review. Adv Chronic Kidney Dis 12: 56–63, 2005 - PubMed
-
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 - PubMed
-
- Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW: Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 3270–3277, 2003 - PubMed
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 - PubMed
-
- Kwan BC, Kronenberg F, Beddhu S, Cheung AK: Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 18: 1246–1261, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

