Accumulation of p100, a precursor of NF-κB2, enhances osteoblastic differentiation in vitro and bone formation in vivo in aly/aly mice
- PMID: 22282470
- PMCID: PMC5417132
- DOI: 10.1210/me.2011-1241
Accumulation of p100, a precursor of NF-κB2, enhances osteoblastic differentiation in vitro and bone formation in vivo in aly/aly mice
Abstract
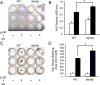
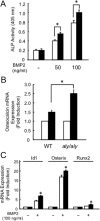
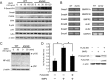
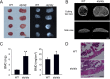
We previously reported that alymphoplasia (aly/aly) mice, which have a natural loss-of-function mutation in the Nik gene, which encodes a kinase essential for the processing of p100 to p52 in the alternative nuclear factor-κB (NF-κB) pathway, show mild osteopetrosis with an increase in several parameters of bone formation: bone formation rate, mineral apposition rate, and osteoblast number. We therefore investigated the molecular mechanisms triggered by the alternative NF-κB pathway in the regulation of osteoblast differentiation using primary osteoblasts (POB) prepared from aly/aly mice. Alkaline phosphatase (ALP) activity and mineralization induced by the presence of β-glycerophosphate and ascorbic acid were enhanced in POB from aly/aly compared with wild-type (WT) mice. Furthermore, osteoblastic differentiation induced by bone morphogenetic protein 2 (BMP2), as shown by ALP activity, mRNA expression of osteocalcin, Id1, Osterix and Runx2, and Sma- and Mad-related protein (Smad)1/5/8 phosphorylation, was also enhanced in POB from aly/aly mice. The ectopic bone formation in vivo that was induced by BMP2 was enhanced in aly/aly mice compared with controls. Transfection of a mutant form of p100, p100ΔGRR, which cannot be processed to p52, stimulated ALP activity and Smad phosphorylation. In contrast to p100ΔGRR, overexpression of p52 inhibited these events. Both BMP2-induced ALP activity and Smad phosphorylation were reduced in POB from p100-deficient mice, which carry a homozygous deletion of the COOH-terminal ankyrin repeats of p100 but still express functional p52 protein. p52 and p100ΔGRR interacted with a BMP receptor, ALK2, in overexpressed COS7 cells and changed the ALK2 protein levels in opposite directions: p52 reduced ALK2 and p100 increased it. Thus, the alternative the NF-κB pathway via the processing of p52 from p100 negatively regulates osteoblastic differentiation and bone formation by modifying BMP activity.
Figures

Similar articles
-
RelB-induced expression of Cot, an MAP3K family member, rescues RANKL-induced osteoclastogenesis in alymphoplasia mice by promoting NF-κB2 processing by IKKα.J Biol Chem. 2014 Mar 14;289(11):7349-61. doi: 10.1074/jbc.M113.538314. Epub 2014 Jan 31. J Biol Chem. 2014. PMID: 24488495 Free PMC article.
-
Processing of the NF-kappa B2 precursor p100 to p52 is critical for RANKL-induced osteoclast differentiation.J Bone Miner Res. 2010 May;25(5):1058-67. doi: 10.1359/jbmr.091032. J Bone Miner Res. 2010. PMID: 19874202
-
The pivotal role of the alternative NF-kappaB pathway in maintenance of basal bone homeostasis and osteoclastogenesis.J Bone Miner Res. 2010 Apr;25(4):809-18. doi: 10.1359/jbmr.091030. J Bone Miner Res. 2010. PMID: 19839765
-
Defective nuclear factor-κB-inducing kinase in aly/aly mice prevents bone resorption induced by local injection of lipopolysaccharide.J Periodontal Res. 2011 Apr;46(2):280-4. doi: 10.1111/j.1600-0765.2010.01333.x. Epub 2010 Dec 29. J Periodontal Res. 2011. PMID: 21348872
-
SCF-mediated degradation of p100 (NF-κB2): mechanisms and relevance in multiple myeloma.Sci Signal. 2012 Dec 4;5(253):pt14. doi: 10.1126/scisignal.2003408. Sci Signal. 2012. PMID: 23211527 Free PMC article. Review.
Cited by
-
Conditional Activation of NF-κB Inducing Kinase (NIK) in the Osteolineage Enhances Both Basal and Loading-Induced Bone Formation.J Bone Miner Res. 2019 Nov;34(11):2087-2100. doi: 10.1002/jbmr.3819. Epub 2019 Jul 31. J Bone Miner Res. 2019. PMID: 31246323 Free PMC article.
-
Inhibition of BMP2-induced bone formation by the p65 subunit of NF-κB via an interaction with Smad4.Mol Endocrinol. 2014 Sep;28(9):1460-70. doi: 10.1210/me.2014-1094. Epub 2014 Jul 16. Mol Endocrinol. 2014. PMID: 25029242 Free PMC article.
-
Conditional loss of IKKα in Osterix + cells has no effect on bone but leads to age-related loss of peripheral fat.Sci Rep. 2022 Mar 22;12(1):4915. doi: 10.1038/s41598-022-08914-6. Sci Rep. 2022. PMID: 35318397 Free PMC article.
-
Inhibition of non-canonical NF-κB signaling suppresses periodontal inflammation and bone loss.Front Immunol. 2023 Apr 18;14:1179007. doi: 10.3389/fimmu.2023.1179007. eCollection 2023. Front Immunol. 2023. PMID: 37143646 Free PMC article.
-
Recent Advances in Mouse Models of Sjögren's Syndrome.Front Immunol. 2020 Jun 30;11:1158. doi: 10.3389/fimmu.2020.01158. eCollection 2020. Front Immunol. 2020. PMID: 32695097 Free PMC article. Review.
References
-
- Aubin JE , Triffitt JT. 2002. Mesenchymal stem cells and osteoblast differentiation. In: , Bilezikian JP , Raisz LG , Rodan GA, eds. Principles of bone biology. 2nd ed San Diego: Academic Press; 59–81
-
- Mundy GR. 1996. Regulation of bone formation by bone morphogenetic proteins and other growth factors. Clin Orthop Relat Res 324:24–28 - PubMed
-
- Yamaguchi A , Komori T , Suda T. 2000. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev 21:393–411 - PubMed
-
- Nishimura R , Hata K , Ikeda F , Ichida F , Shimoyama A , Matsubara T , Wada M , Amano K , Yoneda T. 2008. Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J Bone Miner Metab 26:203–212 - PubMed
-
- Ghosh S , Karin M. 2002. Missing pieces in the NF-κB puzzle. Cell 109:S81–S96 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

