Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma
- PMID: 22262760
- PMCID: PMC3337713
- DOI: 10.1182/blood-2011-10-387365
Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma
Abstract
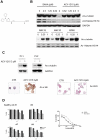
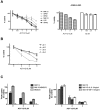
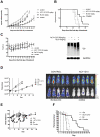
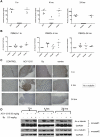
Histone deacetylase (HDAC) enzymatic activity has been linked to the transcription of DNA in cancers including multiple myeloma (MM). Therefore, HDAC inhibitors used alone and in combination are being actively studied as novel therapies in MM. In the present study, we investigated the preclinical activity of ACY-1215, an HDAC6-selective inhibitor, alone and in combination with bortezomib in MM. Low doses of ACY-1215 combined with bortezomib triggered synergistic anti-MM activity, resulting in protracted endoplasmic reticulum stress and apoptosis via activation of caspase-3, caspase-8, and caspase-9 and poly (ADP) ribosome polymerase. In vivo, the anti-MM activity of ACY-1215 in combination with bortezomib was confirmed using 2 different xenograft SCID mouse models: human MM injected subcutaneously (the plasmacytoma model) and luciferase-expressing human MM injected intravenously (the disseminated MM model). Tumor growth was significantly delayed and overall survival was significantly prolonged in animals treated with the combination therapy. Pharmacokinetic data showed peak plasma levels of ACY-1215 at 4 hours after treatment coincident with an increase in acetylated α-tubulin, a marker of HDAC6 inhibition, by immunohistochemistry and Western blot analysis. These studies provide preclinical rationale for acetylated α-tubulin use as a pharmacodynamic biomarker in future clinical trials.
Figures

Similar articles
-
Dual Targeting of Protein Degradation Pathways with the Selective HDAC6 Inhibitor ACY-1215 and Bortezomib Is Synergistic in Lymphoma.Clin Cancer Res. 2015 Oct 15;21(20):4663-75. doi: 10.1158/1078-0432.CCR-14-3068. Epub 2015 Jun 26. Clin Cancer Res. 2015. PMID: 26116270 Free PMC article.
-
Panobinostat synergizes with bortezomib to induce endoplasmic reticulum stress and ubiquitinated protein accumulation in renal cancer cells.BMC Urol. 2014 Aug 30;14:71. doi: 10.1186/1471-2490-14-71. BMC Urol. 2014. PMID: 25176354 Free PMC article.
-
Intracellular NAD⁺ depletion enhances bortezomib-induced anti-myeloma activity.Blood. 2013 Aug 15;122(7):1243-55. doi: 10.1182/blood-2013-02-483511. Epub 2013 Jul 3. Blood. 2013. PMID: 23823317 Free PMC article.
-
Antiproliferative and proapoptotic effects of proteasome inhibitors and their combination with histone deacetylase inhibitors on leukemia cells.Cardiovasc Hematol Disord Drug Targets. 2009 Mar;9(1):62-77. doi: 10.2174/187152909787581372. Cardiovasc Hematol Disord Drug Targets. 2009. PMID: 19275578 Review.
-
Mocetinostat (MGCD0103): a review of an isotype-specific histone deacetylase inhibitor.Expert Opin Investig Drugs. 2011 Jun;20(6):823-9. doi: 10.1517/13543784.2011.577737. Epub 2011 May 10. Expert Opin Investig Drugs. 2011. PMID: 21554162 Free PMC article. Review.
Cited by
-
HDAC6 regulates microtubule stability and clustering of AChRs at neuromuscular junctions.J Cell Biol. 2020 Aug 3;219(8):e201901099. doi: 10.1083/jcb.201901099. J Cell Biol. 2020. PMID: 32697819 Free PMC article.
-
Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin.Clin Cancer Res. 2015 Mar 1;21(5):1161-71. doi: 10.1158/1078-0432.CCR-14-1088. Epub 2014 Dec 26. Clin Cancer Res. 2015. PMID: 25542900 Free PMC article.
-
Cancer epigenetics: new therapies and new challenges.J Drug Deliv. 2013;2013:529312. doi: 10.1155/2013/529312. Epub 2013 Feb 26. J Drug Deliv. 2013. PMID: 23533770 Free PMC article.
-
The role of altered protein acetylation in neurodegenerative disease.Front Aging Neurosci. 2023 Jan 4;14:1025473. doi: 10.3389/fnagi.2022.1025473. eCollection 2022. Front Aging Neurosci. 2023. PMID: 36688174 Free PMC article. Review.
-
Ricolinostat (ACY-1215) suppresses proliferation and promotes apoptosis in esophageal squamous cell carcinoma via miR-30d/PI3K/AKT/mTOR and ERK pathways.Cell Death Dis. 2018 Jul 26;9(8):817. doi: 10.1038/s41419-018-0788-2. Cell Death Dis. 2018. PMID: 30050135 Free PMC article.
References
-
- Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

