Inhibition of the STAT3 signaling pathway is involved in the antitumor activity of cepharanthine in SaOS2 cells
- PMID: 22212432
- PMCID: PMC4010275
- DOI: 10.1038/aps.2011.164
Inhibition of the STAT3 signaling pathway is involved in the antitumor activity of cepharanthine in SaOS2 cells
Abstract
Aim: To investigate the molecular mechanisms underlying the antitumor activity of cepharanthine (CEP), an alkaloid extracted from Stephania cepharantha Hayata.
Methods: Human osteosarcoma cell line SaOS2 was used. MTT assay, Hoechst 33342 nuclear staining, flow cytometry, Western blotting and nude mouse xenografts of SaOS2 cells were applied to examine the antitumor activity of CEP in vitro and in vivo. The expression levels of STAT3 and its downstream signaling molecules were measured with Western blotting and immunochemistry analysis. The activity of STAT3 was detected based on the phosphorylation level of STAT3, luciferase gene reporter assay and translocation of STAT3 to the nucleus.
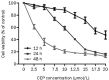
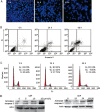
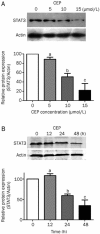
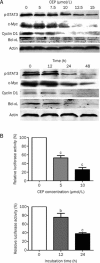
Results: Treatment of SaOS2 cells with CEP (2.5-20 μmol/L) inhibited the cell growth in a concentration- and time-dependent manner. CEP (10 μmol/L) caused cell cycle arrest at G(1) phase and induced apoptosis of SaOS2 cells. CEP (10 and 15 μmol/L) significantly decreased the expression of STAT3 in SaOS2 cells. Furthermore, CEP (5 and 10 μmol/L) significantly inhibited the expression of target genes of STAT3, including the anti-apoptotic gene Bcl-xL and the cell cycle regulators c-Myc and cyclin D1. In nude mouse xenografts of SaOS2 cells, CEP (20 mg·kg(-1)·d(-1), ip for 19 d) significantly reduced the volume and weight of the tumor.
Conclusion: Our findings suggest that inhibition of STAT3 signaling pathway is involved in the anti-tumor activity of CEP.
Figures


Similar articles
-
Inhibition of signal transducer and activator of transcription 3 and cyclooxygenase-2 is involved in radiosensitization of cepharanthine in HeLa cells.Int J Gynecol Cancer. 2013 May;23(4):608-14. doi: 10.1097/IGC.0b013e31828a05fd. Int J Gynecol Cancer. 2013. PMID: 23466568
-
Cryptotanshinone induces cell cycle arrest and apoptosis through the JAK2/STAT3 and PI3K/Akt/NFκB pathways in cholangiocarcinoma cells.Drug Des Devel Ther. 2017 Jun 15;11:1753-1766. doi: 10.2147/DDDT.S132488. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28670110 Free PMC article.
-
Cepharanthine exhibits a potent anticancer activity in p53-mutated colorectal cancer cells through upregulation of p21Waf1/Cip1.Oncol Rep. 2018 Jan;39(1):227-238. doi: 10.3892/or.2017.6084. Epub 2017 Nov 9. Oncol Rep. 2018. PMID: 29138869
-
Therapeutic potential of the biscoclaurine alkaloid, cepharanthine, for a range of clinical conditions.Pharmacol Rep. 2011;63(2):337-47. doi: 10.1016/s1734-1140(11)70500-x. Pharmacol Rep. 2011. PMID: 21602589 Review.
-
Research progress on pharmacological effects and mechanisms of cepharanthine and its derivatives.Naunyn Schmiedebergs Arch Pharmacol. 2023 Nov;396(11):2843-2860. doi: 10.1007/s00210-023-02537-y. Epub 2023 Jun 20. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37338575 Review.
Cited by
-
Cepharanthine suppresses APC-mutant colorectal cancers by down-regulating the expression of β-catenin.Nat Prod Bioprospect. 2024 Feb 29;14(1):18. doi: 10.1007/s13659-024-00443-1. Nat Prod Bioprospect. 2024. PMID: 38421454 Free PMC article.
-
STAT3 and its targeting inhibitors in osteosarcoma.Cell Prolif. 2021 Feb;54(2):e12974. doi: 10.1111/cpr.12974. Epub 2020 Dec 31. Cell Prolif. 2021. PMID: 33382511 Free PMC article. Review.
-
Down-regulation of microRNA-9 leads to activation of IL-6/Jak/STAT3 pathway through directly targeting IL-6 in HeLa cell.Mol Carcinog. 2016 May;55(5):732-42. doi: 10.1002/mc.22317. Epub 2015 Mar 25. Mol Carcinog. 2016. PMID: 25809226 Free PMC article.
-
Target specificity, in vivo pharmacokinetics, and efficacy of the putative STAT3 inhibitor LY5 in osteosarcoma, Ewing's sarcoma, and rhabdomyosarcoma.PLoS One. 2017 Jul 27;12(7):e0181885. doi: 10.1371/journal.pone.0181885. eCollection 2017. PLoS One. 2017. PMID: 28750090 Free PMC article.
-
Eriocalyxin B Inhibits STAT3 Signaling by Covalently Targeting STAT3 and Blocking Phosphorylation and Activation of STAT3.PLoS One. 2015 May 26;10(5):e0128406. doi: 10.1371/journal.pone.0128406. eCollection 2015. PLoS One. 2015. PMID: 26010889 Free PMC article.
References
-
- Kimoto T, Suemitsu K, Nakayama H, Komori E, Ohtani M, Ando S. Therapeutic experience of venomous snakebites by the Japanese viper (Agkistrodon halys Blomhoffii) with low dose of antivenin: report of 43 consecutive cases. Nippon Geka Hokan. 1997;66:71–7. - PubMed
-
- Morita K, Nakamura M, Nagamachi M, Kishi T, Miyachi Y. Seventeen cases of alopecia areata: combination of SADBE topical immunotherapy with other therapies. J Dermatol. 2002;29:661–4. - PubMed
-
- Furusawa S, Wu J. The effects of biscoclaurine alkaloid cepharanthine on mammalian cells: implications for cancer, shock, and inflammatory diseases. Life Sci. 2007;80:1073–9. - PubMed
-
- Goto M, Zeller WP, Hurley RM. Cepharanthine (biscoclaurine alkaloid) treatment in endotoxic shock of suckling rats. J Pharm Pharmacol. 1991;43:589–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

