Th17 cells are long lived and retain a stem cell-like molecular signature
- PMID: 22177921
- PMCID: PMC3246082
- DOI: 10.1016/j.immuni.2011.09.019
Th17 cells are long lived and retain a stem cell-like molecular signature
Abstract
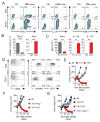
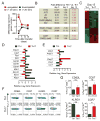
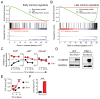
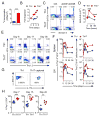
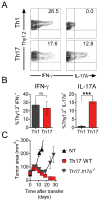
Th17 cells have been described as short lived, but this view is at odds with their capacity to trigger protracted damage to normal and transformed tissues. We report that Th17 cells, despite displaying low expression of CD27 and other phenotypic markers of terminal differentiation, efficiently eradicated tumors and caused autoimmunity, were long lived, and maintained a core molecular signature resembling early memory CD8(+) cells with stem cell-like properties. In addition, we found that Th17 cells had high expression of Tcf7, a direct target of the Wnt and β-catenin signaling axis, and accumulated β-catenin, a feature observed in stem cells. In vivo, Th17 cells gave rise to Th1-like effector cell progeny and also self-renewed and persisted as IL-17A-secreting cells. Multipotency was required for Th17 cell-mediated tumor eradication because effector cells deficient in IFN-γ or IL-17A had impaired activity. Thus, Th17 cells are not always short lived and are a less-differentiated subset capable of superior persistence and functionality.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Stem-cell-like qualities of immune memory; CD4+ T cells join the party.Cell Stem Cell. 2012 Feb 3;10(2):107-8. doi: 10.1016/j.stem.2012.01.011. Cell Stem Cell. 2012. PMID: 22305557 Free PMC article.
Similar articles
-
Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity.Nature. 2019 Jan;565(7737):101-105. doi: 10.1038/s41586-018-0806-7. Epub 2018 Dec 19. Nature. 2019. PMID: 30568299 Free PMC article.
-
IL-7/IL-7 Receptor Signaling Differentially Affects Effector CD4+ T Cell Subsets Involved in Experimental Autoimmune Encephalomyelitis.J Immunol. 2015 Sep 1;195(5):1974-83. doi: 10.4049/jimmunol.1403135. Epub 2015 Jul 29. J Immunol. 2015. PMID: 26223651 Free PMC article.
-
Involvement of CD26 in Differentiation and Functions of Th1 and Th17 Subpopulations of T Lymphocytes.J Immunol Res. 2021 Jan 20;2021:6671410. doi: 10.1155/2021/6671410. eCollection 2021. J Immunol Res. 2021. PMID: 33542930 Free PMC article.
-
The pathogenicity of Th17 cells in autoimmune diseases.Semin Immunopathol. 2019 May;41(3):283-297. doi: 10.1007/s00281-019-00733-8. Epub 2019 Mar 19. Semin Immunopathol. 2019. PMID: 30891627 Review.
-
Th17 cells in immunity and autoimmunity.Clin Dev Immunol. 2013;2013:986789. doi: 10.1155/2013/986789. Epub 2013 Dec 26. Clin Dev Immunol. 2013. PMID: 24454481 Free PMC article. Review.
Cited by
-
The Th17 family: flexibility follows function.Immunol Rev. 2013 Mar;252(1):89-103. doi: 10.1111/imr.12035. Immunol Rev. 2013. PMID: 23405897 Free PMC article. Review.
-
Programming Multifaceted Pulmonary T Cell Immunity by Combination Adjuvants.Cell Rep Med. 2020 Sep 22;1(6):100095. doi: 10.1016/j.xcrm.2020.100095. Cell Rep Med. 2020. PMID: 32984856 Free PMC article.
-
Differentiation of distinct long-lived memory CD4 T cells in intestinal tissues after oral Listeria monocytogenes infection.Mucosal Immunol. 2017 Mar;10(2):520-530. doi: 10.1038/mi.2016.66. Epub 2016 Jul 27. Mucosal Immunol. 2017. PMID: 27461178 Free PMC article.
-
Chronic dry eye disease is principally mediated by effector memory Th17 cells.Mucosal Immunol. 2014 Jan;7(1):38-45. doi: 10.1038/mi.2013.20. Epub 2013 Apr 10. Mucosal Immunol. 2014. PMID: 23571503 Free PMC article.
-
Distinguishing between help and harm: Helper T cell subsets and immune-related adverse events.J Clin Invest. 2024 Oct 15;134(20):e184310. doi: 10.1172/JCI184310. J Clin Invest. 2024. PMID: 39403930 Free PMC article.
References
-
- Ballesteros-Tato A, Randall Troy D. Memory: The Incomplete Unhappening of Differentiation. Immunity. 2011;35:496–498. - PubMed
-
- Benoist C, Germain RN, Mathis D. A plaidoyer for ‘systems immunology’. Immunological reviews. 2006;210:229–234. - PubMed
-
- Beyersdorf N, Ding X, Tietze JK, Hanke T. Characterization of mouse CD4 T cell subsets defined by expression of KLRG1. European journal of immunology. 2007;37:3445–3454. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

