CD11b⁺, Ly6G⁺ cells produce type I interferon and exhibit tissue protective properties following peripheral virus infection
- PMID: 22102816
- PMCID: PMC3213107
- DOI: 10.1371/journal.ppat.1002374
CD11b⁺, Ly6G⁺ cells produce type I interferon and exhibit tissue protective properties following peripheral virus infection
Abstract
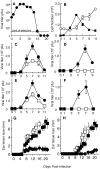
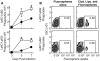
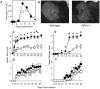
The goal of the innate immune system is containment of a pathogen at the site of infection prior to the initiation of an effective adaptive immune response. However, effector mechanisms must be kept in check to combat the pathogen while simultaneously limiting undesirable destruction of tissue resulting from these actions. Here we demonstrate that innate immune effector cells contain a peripheral poxvirus infection, preventing systemic spread of the virus. These innate immune effector cells are comprised primarily of CD11b⁺Ly6C⁺Ly6G⁻ monocytes that accumulate initially at the site of infection, and are then supplemented and eventually replaced by CD11b⁺Ly6C⁺Ly6G⁺ cells. The phenotype of the CD11b⁺Ly6C⁺Ly6G⁺ cells resembles neutrophils, but the infiltration of neutrophils typically occurs prior to, rather than following, accumulation of monocytes. Indeed, it appears that the CD11b⁺Ly6C⁺Ly6G⁺ cells that infiltrated the site of VACV infection in the ear are phenotypically distinct from the classical description of both neutrophils and monocyte/macrophages. We found that CD11b⁺Ly6C⁺Ly6G⁺ cells produce Type I interferons and large quantities of reactive oxygen species. We also observed that depletion of Ly6G⁺ cells results in a dramatic increase in tissue damage at the site of infection. Tissue damage is also increased in the absence of reactive oxygen species, although reactive oxygen species are typically thought to be damaging to tissue rather than protective. These data indicate the existence of a specialized population of CD11b⁺Ly6C⁺Ly6G⁺ cells that infiltrates a site of virus infection late and protects the infected tissue from immune-mediated damage via production of reactive oxygen species. Regulation of the action of this population of cells may provide an intervention to prevent innate immune-mediated tissue destruction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Type I interferon-dependent CCL4 is induced by a cGAS/STING pathway that bypasses viral inhibition and protects infected tissue, independent of viral burden.PLoS Pathog. 2019 Oct 11;15(10):e1007778. doi: 10.1371/journal.ppat.1007778. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31603920 Free PMC article.
-
A systemic macrophage response is required to contain a peripheral poxvirus infection.PLoS Pathog. 2017 Jun 14;13(6):e1006435. doi: 10.1371/journal.ppat.1006435. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28614386 Free PMC article.
-
Anatomically restricted synergistic antiviral activities of innate and adaptive immune cells in the skin.Cell Host Microbe. 2013 Feb 13;13(2):155-68. doi: 10.1016/j.chom.2013.01.004. Cell Host Microbe. 2013. PMID: 23414756 Free PMC article.
-
Vaccinia Virus Protein C6: A Multifunctional Interferon Antagonist.Adv Exp Med Biol. 2018;1052:1-7. doi: 10.1007/978-981-10-7572-8_1. Adv Exp Med Biol. 2018. PMID: 29785476 Review.
-
Evasion of innate immunity by vaccinia virus.Parasitology. 2005;130 Suppl:S11-25. doi: 10.1017/S0031182005008127. Parasitology. 2005. PMID: 16281988 Review.
Cited by
-
An Immature Myeloid/Myeloid-Suppressor Cell Response Associated with Necrotizing Inflammation Mediates Lethal Pulmonary Tularemia.PLoS Pathog. 2016 Mar 25;12(3):e1005517. doi: 10.1371/journal.ppat.1005517. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 27015566 Free PMC article.
-
T Cell Surveillance during Cutaneous Viral Infections.Viruses. 2024 Apr 26;16(5):679. doi: 10.3390/v16050679. Viruses. 2024. PMID: 38793562 Free PMC article. Review.
-
Mpox (formerly monkeypox): pathogenesis, prevention, and treatment.Signal Transduct Target Ther. 2023 Dec 27;8(1):458. doi: 10.1038/s41392-023-01675-2. Signal Transduct Target Ther. 2023. PMID: 38148355 Free PMC article. Review.
-
Human Neutrophils Present Mild Activation by Zika Virus But Reduce the Infection of Susceptible Cells.Front Immunol. 2022 Jun 7;13:784443. doi: 10.3389/fimmu.2022.784443. eCollection 2022. Front Immunol. 2022. PMID: 35747137 Free PMC article.
-
The prospective outcome of the monkeypox outbreak in 2022 and characterization of monkeypox disease immunobiology.Front Cell Infect Microbiol. 2023 Jul 18;13:1196699. doi: 10.3389/fcimb.2023.1196699. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37533932 Free PMC article.
References
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
-
- Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. - PubMed
-
- Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7:1201–1214. - PubMed
-
- Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005;5:905–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

