Both the unique and repeat regions of the Porphyromonas gingivalis hemagglutin A are involved in adhesion and invasion of host cells
- PMID: 22100486
- PMCID: PMC3278541
- DOI: 10.1016/j.anaerobe.2011.10.005
Both the unique and repeat regions of the Porphyromonas gingivalis hemagglutin A are involved in adhesion and invasion of host cells
Abstract
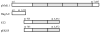
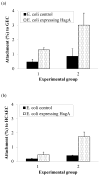
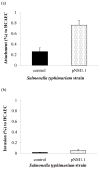
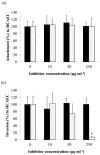
Porphyromonas gingivalis is one of the major etiologic agents of adult periodontitis and has been associated with cardiovascular diseases. It expresses multiple hemagglutinins that are significant virulence factors and play an important role in bacterial attachment and invasion of host cells. The objective of this study was to determine the impact of P. gingivalis hemagglutinin A (HagA) on the attachment to and invasion of human coronary artery endothelial cells (HCAEC) and gingival epithelial cells (GEC). Bacterial strains expressing the HagA protein (or subunits), including Escherichia coli carrying plasmid pEKS5, E. coli carrying plasmid ST2, and Salmonella enterica serovar Typhimurium with plasmid pNM1.1 were used in this study. The strains were tested for their ability to attach to and invade HCAEC and GEC using antibiotic protection assays. In addition, the unique 5' N-terminal non-repeated segment of HagA was purified in recombinant form and a monoclonal antibody was created against the polypeptide. The monoclonal antibody against the unique portion of HagA was tested for inhibitory activity in these assays. The attachment of both E. coli strains expressing HagA fragment to host cells was significantly increased compared to their respective controls. However, they did not invade GEC or HCAEC. Interestingly, HagA expression in the Salmonella strain increased both adherence to and invasion of HCAEC, which may be due to the presence of the entire hagA ORF. A monoclonal antibody against the unique 5' N-terminal portion of HagA reduced invasion. Further experiments are needed to determine the role of the unique and the repeat segments of P. gingivalis HagA.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Expression and immunogenicity of hemagglutinin A from Porphyromonas gingivalis in an avirulent Salmonella enterica serovar typhimurium vaccine strain.Infect Immun. 2000 Feb;68(2):732-9. doi: 10.1128/IAI.68.2.732-739.2000. Infect Immun. 2000. PMID: 10639440 Free PMC article.
-
Subcloning of the 200-kDa Porphyromonas gingivalis antigen gene and inhibition of hemagglutination by an antibody against the recombinant protein.J Oral Sci. 2004 Sep;46(3):163-9. doi: 10.2334/josnusd.46.163. J Oral Sci. 2004. PMID: 15508749
-
Porphyromonas gingivalis virulence factors and invasion of cells of the cardiovascular system.J Periodontal Res. 1999 Oct;34(7):393-9. doi: 10.1111/j.1600-0765.1999.tb02272.x. J Periodontal Res. 1999. PMID: 10685367
-
Porphyromonas gingivalis gingipains: the molecular teeth of a microbial vampire.Curr Protein Pept Sci. 2003 Dec;4(6):409-26. doi: 10.2174/1389203033487009. Curr Protein Pept Sci. 2003. PMID: 14683427 Review.
-
The role of gingipains in the pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):111-8. doi: 10.1902/jop.2003.74.1.111. J Periodontol. 2003. PMID: 12593605 Review.
Cited by
-
Exploring the presence of oral bacteria in non-oral sites of patients with cardiovascular diseases using whole metagenomic data.Sci Rep. 2024 Jan 17;14(1):1476. doi: 10.1038/s41598-023-50891-x. Sci Rep. 2024. PMID: 38233502 Free PMC article.
-
Quercetin inhibits virulence properties of Porphyromas gingivalis in periodontal disease.Sci Rep. 2020 Oct 27;10(1):18313. doi: 10.1038/s41598-020-74977-y. Sci Rep. 2020. PMID: 33110205 Free PMC article.
-
Biogenesis and function of Porphyromonas gingivalis outer membrane vesicles.Future Microbiol. 2015;10(9):1517-27. doi: 10.2217/fmb.15.63. Epub 2015 Sep 7. Future Microbiol. 2015. PMID: 26343879 Free PMC article. Review.
-
Deletion of lipoprotein PG0717 in Porphyromonas gingivalis W83 reduces gingipain activity and alters trafficking in and response by host cells.PLoS One. 2013 Sep 12;8(9):e74230. doi: 10.1371/journal.pone.0074230. eCollection 2013. PLoS One. 2013. PMID: 24069284 Free PMC article.
-
Variability in Genomic and Virulent Properties of Porphyromonas gingivalis Strains Isolated From Healthy and Severe Chronic Periodontitis Individuals.Front Cell Infect Microbiol. 2019 Jul 10;9:246. doi: 10.3389/fcimb.2019.00246. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31355151 Free PMC article.
References
-
- Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. - PubMed
-
- Page RC. The etiology and pathogenesis of periodontitis. Compend Contin Educ Dent. 2002;23:11–4. - PubMed
-
- Slots J, Listgarten MA. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. - PubMed
-
- Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999;20:82–121. - PubMed
-
- Slots J, Bragd L, Wikström M, Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

