Rapamycin with antiretroviral therapy in AIDS-associated Kaposi sarcoma: an AIDS Malignancy Consortium study
- PMID: 22067664
- PMCID: PMC3302934
- DOI: 10.1097/QAI.0b013e31823e7884
Rapamycin with antiretroviral therapy in AIDS-associated Kaposi sarcoma: an AIDS Malignancy Consortium study
Abstract
Purpose: The mammalian target of rapamycin is activated in Kaposi sarcoma (KS) and its inhibitor, rapamycin, has induced KS regression in transplant-associated KS. This study aimed to evaluate rapamycin's safety and toxicity in HIV-infected individuals with KS receiving antiretroviral therapy (ART), investigate rapamycin interactions with both protease inhibitor (PI)-containing and nonnucleoside reverse transcriptase inhibitor (NNRTI)-containing ART regimens, and assess clinical and biological endpoints including KS response and mammalian target of rapamycin-dependent signaling.
Methods: Seven participants, 4 on PI-based and 3 on NNRTI-based ART, had rapamycin titrated to achieve trough concentrations of 5-10 ng/mL. Patients were monitored for safety and KS response. KS biopsies were evaluated for changes in phosphoribosomal S6 protein, and phospho-Akt expression. Interleukin 6 and vascular endothelial growth factor levels, HIV and KS-associated herpesvirus viral loads, and CD4 counts were monitored.
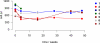
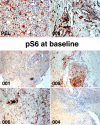
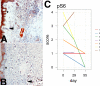
Results: Despite pharmacokinetic interactions resulting in >200-fold differences in cumulative weekly rapamycin doses between participants on PI-containing and NNRTI-containing regimens, treatment was well tolerated. There were no significant changes in viral loads or cytokine levels; modest initial decreases in CD4 counts occurred in some patients. Three participants, all on PI-containing regimens and with higher rapamycin exposure, showed partial KS responses. Three of 4 subjects whose biopsies were studied at ≥day 50 showed decreased phosphoribosomal S6 protein staining.
Conclusions: Rapamycin seems safe in HIV-infected individuals with KS and can, in some cases, induce tumor regression and affect its molecular targets. Significant pharmacokinetic interactions require careful titration to achieve target drug trough concentrations but may be exploited to achieve therapeutic benefit.
Figures
Similar articles
-
Clinical outcomes of HIV-infected patients with Kaposi's sarcoma receiving nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy in Uganda.HIV Med. 2012 Mar;13(3):166-71. doi: 10.1111/j.1468-1293.2011.00955.x. Epub 2011 Nov 24. HIV Med. 2012. PMID: 22112164
-
Prospective study of the effects of antiretroviral therapy on Kaposi sarcoma--associated herpesvirus infection in patients with and without Kaposi sarcoma.J Acquir Immune Defic Syndr. 2002 Dec 1;31(4):384-90. doi: 10.1097/00126334-200212010-00003. J Acquir Immune Defic Syndr. 2002. PMID: 12447008
-
Remission from Kaposi's sarcoma on HAART is associated with suppression of HIV replication and is independent of protease inhibitor therapy.Br J Cancer. 2006 Apr 10;94(7):1000-6. doi: 10.1038/sj.bjc.6603056. Br J Cancer. 2006. PMID: 16570046 Free PMC article.
-
Akt/TSC/mTOR activation by the KSHV G protein-coupled receptor: emerging insights into the molecular oncogenesis and treatment of Kaposi's sarcoma.Cell Cycle. 2007 Feb 15;6(4):438-43. doi: 10.4161/cc.6.4.3843. Epub 2007 Feb 12. Cell Cycle. 2007. PMID: 17329974 Review.
-
Treatment of AIDS-related Kaposi's sarcoma with interleukin-12: rationale and preliminary evidence of clinical activity.Crit Rev Immunol. 2007;27(5):401-14. doi: 10.1615/critrevimmunol.v27.i5.10. Crit Rev Immunol. 2007. PMID: 18197804 Review.
Cited by
-
HIV-associated chronic immune activation.Immunol Rev. 2013 Jul;254(1):78-101. doi: 10.1111/imr.12079. Immunol Rev. 2013. PMID: 23772616 Free PMC article. Review.
-
Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy.J Clin Invest. 2016 Sep 1;126(9):3165-75. doi: 10.1172/JCI84418. Epub 2016 Sep 1. J Clin Invest. 2016. PMID: 27584730 Free PMC article. Review.
-
Diagnosis and Treatment of Kaposi Sarcoma.Am J Clin Dermatol. 2017 Aug;18(4):529-539. doi: 10.1007/s40257-017-0270-4. Am J Clin Dermatol. 2017. PMID: 28324233 Free PMC article. Review.
-
Plasma biomarkers of clinical response during chemotherapy plus combination antiretroviral therapy (cART) in HIV+ patients with advanced Kaposi sarcoma.Oncotarget. 2015 Oct 6;6(30):30334-42. doi: 10.18632/oncotarget.4571. Oncotarget. 2015. PMID: 26296972 Free PMC article.
-
Immune Reconstitution Inflammatory Syndrome Associated Kaposi Sarcoma.Cancers (Basel). 2022 Feb 16;14(4):986. doi: 10.3390/cancers14040986. Cancers (Basel). 2022. PMID: 35205734 Free PMC article. Review.
References
-
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007 May 31;356(22):2271–2281. - PubMed
-
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010 Sep 15;116(18):4256–4265. - PubMed
-
- Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009 Aug 10;27(23):3822–3829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

