Lymph node stromal cells enhance drug-resistant colon cancer cell tumor formation through SDF-1α/CXCR4 paracrine signaling
- PMID: 21969820
- PMCID: PMC3182279
- DOI: 10.1593/neo.11324
Lymph node stromal cells enhance drug-resistant colon cancer cell tumor formation through SDF-1α/CXCR4 paracrine signaling
Abstract
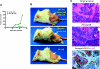
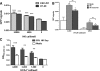
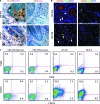
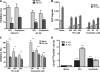
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related deaths in America. Nearly two thirds of newly diagnosed CRC cases include lymph node (LN) involvement, and LN metastasis is one of the strongest negative prognostic factors for CRC. It is thought that CRC tumors contain a small population of drug-resistant CRC tumor-initiating cells (Co-TICs) that may be responsible for cancer recurrence. To evaluate the effects of the LN stromal cells on Co-TICs, we established a unique xenoplant model using CRC cells isolated by enzymatic digestion from consented patient specimens, HT-29 cells, HCA-7 cells, and LN stromal cell line HK cells. We found that HK cells and HK cell-conditioned media enhanced CRC tumor formation and tumor angiogenesis. Cells expressing CD133(+) and the stromal cell-derived factor 1α (SDF-1α) receptor CXCR4 were enriched in chemotherapeutic-resistant CRC cells. CD133(+)CXCR4(+) Co-TICs isolated from patient specimens are more tumorigenic than unsorted tumor cells. Furthermore, the inhibitors specific to HK cell-derived SDF-1α reduced tumor formation and tumor angiogenesis. Our results have demonstrated a role for Co-TICs in tumor growth and defined the influence of LN stromal cells on Co-TICs. We have identified a major Co-TIC/LN microenvironment-specific mechanism for CRC resistance to chemotherapeutic agents and established experimental platforms for both in vitro and in vivo testing, indicating that SDF-1α and its receptor, CXCR4, may be targets for clinical therapy.
Figures


Similar articles
-
The critical roles of tumor-initiating cells and the lymph node stromal microenvironment in human colorectal cancer extranodal metastasis using a unique humanized orthotopic mouse model.FASEB J. 2015 Aug;29(8):3571-81. doi: 10.1096/fj.14-268938. Epub 2015 May 11. FASEB J. 2015. PMID: 25962655
-
CD 133+ and CXCR4+ colon cancer cells as a marker for lymph node metastasis.J Surg Res. 2013 Nov;185(1):113-8. doi: 10.1016/j.jss.2013.05.049. Epub 2013 Jun 5. J Surg Res. 2013. PMID: 23777983
-
CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche.Cancer Res. 2010 Dec 15;70(24):10411-21. doi: 10.1158/0008-5472.CAN-10-2591. Epub 2010 Nov 5. Cancer Res. 2010. PMID: 21056990
-
Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice.Exp Hematol. 2006 Aug;34(8):967-75. doi: 10.1016/j.exphem.2006.04.002. Exp Hematol. 2006. PMID: 16863903 Review.
-
CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment.Blood. 2006 Mar 1;107(5):1761-7. doi: 10.1182/blood-2005-08-3182. Epub 2005 Nov 3. Blood. 2006. PMID: 16269611 Review.
Cited by
-
CD133: a cancer stem cells marker, is used in colorectal cancers.World J Gastroenterol. 2013 May 7;19(17):2603-11. doi: 10.3748/wjg.v19.i17.2603. World J Gastroenterol. 2013. PMID: 23674867 Free PMC article. Review.
-
CXC family of chemokines as prognostic or predictive biomarkers and possible drug targets in colorectal cancer.World J Gastroenterol. 2018 Nov 14;24(42):4738-4749. doi: 10.3748/wjg.v24.i42.4738. World J Gastroenterol. 2018. PMID: 30479461 Free PMC article. Review.
-
Regulation of signal transduction pathways in colorectal cancer: implications for therapeutic resistance.PeerJ. 2021 Oct 22;9:e12338. doi: 10.7717/peerj.12338. eCollection 2021. PeerJ. 2021. PMID: 34733591 Free PMC article.
-
Chemotherapy induces adaptive drug resistance and metastatic potentials via phenotypic CXCR4-expressing cell state transition in ovarian cancer.PLoS One. 2017 Feb 14;12(2):e0171044. doi: 10.1371/journal.pone.0171044. eCollection 2017. PLoS One. 2017. PMID: 28196146 Free PMC article.
-
Colorectal Cancer: The Contribution of CXCL12 and Its Receptors CXCR4 and CXCR7.Cancers (Basel). 2022 Apr 2;14(7):1810. doi: 10.3390/cancers14071810. Cancers (Basel). 2022. PMID: 35406582 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. - PubMed
-
- SEER Cancer Statistics Review. [April 18, 2011]. Available at: http://seer.cancer.gov/statfacts/html/colorect.html.
-
- Nakagawa H, Liyanarachchi S, Davuluri RV, Auer H, Martin EW, Jr, de la Chapelle A, Frankel WL. Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene. 2004;23:7366–7377. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
