Luciferase-expressing Leishmania infantum allows the monitoring of amastigote population size, in vivo, ex vivo and in vitro
- PMID: 21931877
- PMCID: PMC3172198
- DOI: 10.1371/journal.pntd.0001323
Luciferase-expressing Leishmania infantum allows the monitoring of amastigote population size, in vivo, ex vivo and in vitro
Abstract
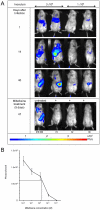
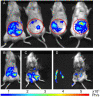
Here we engineered transgenic Leishmania infantum that express luciferase, the objectives being to more easily monitor in real time their establishment either in BALB/c mice--the liver and spleen being mainly studied-or in vitro. Whatever stationary phase L. infantum promastigotes population--wild type or engineered to express luciferase-the parasite burden was similar in the liver and the spleen at day 30 post the intravenous inoculation of BALB/c mice. Imaging of L. infantum hosting BALB/C mice provided sensitivity in the range of 20,000 to 40,000 amastigotes/mg tissue, two tissues-liver and spleen-being monitored. Once sampled and processed ex vivo for their luciferin-dependent bioluminescence the threshold sensitivity was shown to range from 1,000 to 6,000 amastigotes/mg tissue. This model further proved to be valuable for in vivo measurement of the efficiency of drugs such as miltefosine and may, therefore, additionally be used to evaluate vaccine-induced protection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Generation of luciferase-expressing Leishmania infantum chagasi and assessment of miltefosine efficacy in infected hamsters through bioimaging.PLoS Negl Trop Dis. 2015 Feb 13;9(2):e0003556. doi: 10.1371/journal.pntd.0003556. eCollection 2015 Feb. PLoS Negl Trop Dis. 2015. PMID: 25679212 Free PMC article.
-
Potentiation of the leishmanicidal activity of nelfinavir in combination with miltefosine or amphotericin B.Int J Antimicrob Agents. 2018 Nov;52(5):682-687. doi: 10.1016/j.ijantimicag.2018.06.016. Epub 2018 Jun 30. Int J Antimicrob Agents. 2018. PMID: 29969693
-
A chronic bioluminescent model of experimental visceral leishmaniasis for accelerating drug discovery.PLoS Negl Trop Dis. 2019 Feb 14;13(2):e0007133. doi: 10.1371/journal.pntd.0007133. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30763330 Free PMC article.
-
Acarbose presents in vitro and in vivo antileishmanial activity against Leishmania infantum and is a promising therapeutic candidate against visceral leishmaniasis.Med Microbiol Immunol. 2021 Jun;210(2-3):133-147. doi: 10.1007/s00430-021-00707-4. Epub 2021 Apr 18. Med Microbiol Immunol. 2021. PMID: 33870453 Free PMC article.
-
In vitro and in vivo antileishmanial activity of a fluoroquinoline derivate against Leishmania infantum and Leishmania amazonensis species.Acta Trop. 2019 Mar;191:29-37. doi: 10.1016/j.actatropica.2018.12.036. Epub 2018 Dec 23. Acta Trop. 2019. PMID: 30586571
Cited by
-
Synthesis of Nitrostyrylthiazolidine-2,4-dione Derivatives Displaying Antileishmanial Potential.Pharmaceuticals (Basel). 2024 Jul 3;17(7):878. doi: 10.3390/ph17070878. Pharmaceuticals (Basel). 2024. PMID: 39065730 Free PMC article.
-
Development of Environmentally Responsive Self-Emulsifying System Containing Copaiba Oil-Resin for Leishmaniasis Oral Treatment.Pharmaceutics. 2023 Aug 12;15(8):2127. doi: 10.3390/pharmaceutics15082127. Pharmaceutics. 2023. PMID: 37631341 Free PMC article.
-
Leishmania Animal Models Used in Drug Discovery: A Systematic Review.Animals (Basel). 2023 May 16;13(10):1650. doi: 10.3390/ani13101650. Animals (Basel). 2023. PMID: 37238080 Free PMC article. Review.
-
Beyond energy balance regulation: The underestimated role of adipose tissues in host defense against pathogens.Front Immunol. 2023 Mar 2;14:1083191. doi: 10.3389/fimmu.2023.1083191. eCollection 2023. Front Immunol. 2023. PMID: 36936928 Free PMC article. Review.
-
Comparison of Bioluminescent Substrates in Natural Infection Models of Neglected Parasitic Diseases.Int J Mol Sci. 2022 Dec 16;23(24):16074. doi: 10.3390/ijms232416074. Int J Mol Sci. 2022. PMID: 36555716 Free PMC article.
References
-
- Ridley RG. Evaluating diagnostics: VL. Nat Rev Micro 2007
-
- Sereno D, Cordeiro da Silva A, Mathieu-Daude F, Ouaissi A. Advances and perspectives in Leishmania cell based drug-screening procedures. Parasitol Int. 2007;56:3–7. - PubMed
-
- Chan MM, Bulinski JC, Chang KP, Fong D. A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitol Res. 2003;89:266–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

