Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells
- PMID: 21930770
- PMCID: PMC3182051
- DOI: 10.1084/jem.20101956
Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells
Abstract
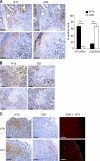
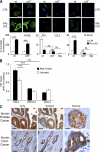
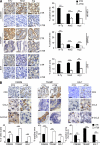
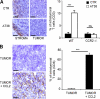
Tumor-promoted constraints negatively affect cytotoxic T lymphocyte (CTL) trafficking to the tumor core and, as a result, inhibit tumor killing. The production of reactive nitrogen species (RNS) within the tumor microenvironment has been reported in mouse and human cancers. We describe a novel RNS-dependent posttranslational modification of chemokines that has a profound impact on leukocyte recruitment to mouse and human tumors. Intratumoral RNS production induces CCL2 chemokine nitration and hinders T cell infiltration, resulting in the trapping of tumor-specific T cells in the stroma that surrounds cancer cells. Preconditioning of the tumor microenvironment with novel drugs that inhibit CCL2 modification facilitates CTL invasion of the tumor, suggesting that these drugs may be effective in cancer immunotherapy. Our results unveil an unexpected mechanism of tumor evasion and introduce new avenues for cancer immunotherapy.
Figures


Similar articles
-
Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds.J Immunol. 2013 Jun 15;190(12):6673-80. doi: 10.4049/jimmunol.1300434. Epub 2013 May 17. J Immunol. 2013. PMID: 23686489
-
Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10.J Immunother Cancer. 2020 May;8(1):e000599. doi: 10.1136/jitc-2020-000599. J Immunother Cancer. 2020. PMID: 32461349 Free PMC article.
-
Interrupting the nitrosative stress fuels tumor-specific cytotoxic T lymphocytes in pancreatic cancer.J Immunother Cancer. 2022 Jan;10(1):e003549. doi: 10.1136/jitc-2021-003549. J Immunother Cancer. 2022. PMID: 35022194 Free PMC article.
-
Improving homing in T cell therapy.Cytokine Growth Factor Rev. 2017 Aug;36:107-116. doi: 10.1016/j.cytogfr.2017.06.009. Epub 2017 Jun 23. Cytokine Growth Factor Rev. 2017. PMID: 28690108 Review.
-
Hiding the road signs that lead to tumor immunity.J Exp Med. 2011 Sep 26;208(10):1937-40. doi: 10.1084/jem.20111856. J Exp Med. 2011. PMID: 21948803 Free PMC article. Review.
Cited by
-
Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment.Oxid Med Cell Longev. 2016;2016:1580967. doi: 10.1155/2016/1580967. Epub 2016 Jul 28. Oxid Med Cell Longev. 2016. PMID: 27547291 Free PMC article. Review.
-
Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy.Oncoimmunology. 2015 Feb 3;4(1):e954829. doi: 10.4161/21624011.2014.954829. eCollection 2015 Jan. Oncoimmunology. 2015. PMID: 25949858 Free PMC article. Review.
-
Trial Watch-Small molecules targeting the immunological tumor microenvironment for cancer therapy.Oncoimmunology. 2016 Mar 10;5(6):e1149674. doi: 10.1080/2162402X.2016.1149674. eCollection 2016 Jun. Oncoimmunology. 2016. PMID: 27471617 Free PMC article. Review.
-
Repression of MUC1 Promotes Expansion and Suppressive Function of Myeloid-Derived Suppressor Cells in Pancreatic and Breast Cancer Murine Models.Int J Mol Sci. 2021 May 25;22(11):5587. doi: 10.3390/ijms22115587. Int J Mol Sci. 2021. PMID: 34070449 Free PMC article.
-
Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression.Front Immunol. 2018 Oct 30;9:2499. doi: 10.3389/fimmu.2018.02499. eCollection 2018. Front Immunol. 2018. PMID: 30425715 Free PMC article. Review.
References
-
- Apolloni E., Bronte V., Mazzoni A., Serafini P., Cabrelle A., Segal D.M., Young H.A., Zanovello P. 2000. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J. Immunol. 165:6723–6730 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

