Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice
- PMID: 21911941
- PMCID: PMC3195459
- DOI: 10.1172/JCI45862
Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice
Abstract
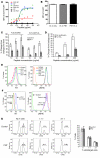
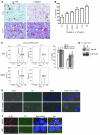
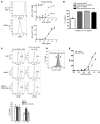
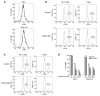
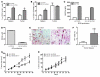
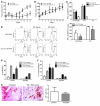
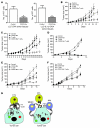
Cancer immunotherapeutic approaches induce tumor-specific immune responses, in particular CTL responses, in many patients treated. However, such approaches are clinically beneficial to only a few patients. We set out to investigate one possible explanation for the failure of CTLs to eliminate tumors, specifically, the concept that this failure is not dependent on inhibition of T cell function. In a previous study, we found that in mice, myeloid-derived suppressor cells (MDSCs) are a source of the free radical peroxynitrite (PNT). Here, we show that pre-treatment of mouse and human tumor cells with PNT or with MDSCs inhibits binding of processed peptides to tumor cell-associated MHC, and as a result, tumor cells become resistant to antigen-specific CTLs. This effect was abrogated in MDSCs treated with a PNT inhibitor. In a mouse model of tumor-associated inflammation in which the antitumor effects of antigen-specific CTLs are eradicated by expression of IL-1β in the tumor cells, we determined that therapeutic failure was not caused by more profound suppression of CTLs by IL-1β-expressing tumors than tumors not expressing this proinflammatory cytokine. Rather, therapeutic failure was a result of the presence of PNT. Clinical relevance for these data was suggested by the observation that myeloid cells were the predominant source of PNT in human lung, pancreatic, and breast cancer samples. Our data therefore suggest what we believe to be a novel mechanism of MDSC-mediated tumor cell resistance to CTLs.
Figures

Similar articles
-
IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function.Eur J Immunol. 2010 Dec;40(12):3347-57. doi: 10.1002/eji.201041037. Eur J Immunol. 2010. PMID: 21110318 Free PMC article.
-
Peroxynitrite in the tumor microenvironment changes the profile of antigens allowing escape from cancer immunotherapy.Cancer Cell. 2022 Oct 10;40(10):1173-1189.e6. doi: 10.1016/j.ccell.2022.09.001. Cancer Cell. 2022. PMID: 36220073 Free PMC article.
-
Fas signal promotes lung cancer growth by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2.J Immunol. 2009 Mar 15;182(6):3801-8. doi: 10.4049/jimmunol.0801548. J Immunol. 2009. PMID: 19265159
-
CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review.J Cell Physiol. 2019 Jun;234(6):8509-8521. doi: 10.1002/jcp.27782. Epub 2018 Nov 22. J Cell Physiol. 2019. PMID: 30520029 Review.
-
Myeloid suppressor cells and immune modulation in lung cancer.Immunotherapy. 2012 Mar;4(3):291-304. doi: 10.2217/imt.11.178. Immunotherapy. 2012. PMID: 22401635 Free PMC article. Review.
Cited by
-
Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy.J Hematol Oncol. 2022 Aug 28;15(1):118. doi: 10.1186/s13045-022-01335-y. J Hematol Oncol. 2022. PMID: 36031601 Free PMC article. Review.
-
Identification of CDK1, PBK, and CHEK1 as an Oncogenic Signature in Glioblastoma: A Bioinformatics Approach to Repurpose Dapagliflozin as a Therapeutic Agent.Int J Mol Sci. 2023 Nov 16;24(22):16396. doi: 10.3390/ijms242216396. Int J Mol Sci. 2023. PMID: 38003585 Free PMC article.
-
The immune system and inflammation in breast cancer.Mol Cell Endocrinol. 2014 Jan 25;382(1):673-682. doi: 10.1016/j.mce.2013.06.003. Epub 2013 Jun 19. Mol Cell Endocrinol. 2014. PMID: 23791814 Free PMC article. Review.
-
Nanotechnology Applications in Breast Cancer Immunotherapy.Small. 2024 Oct;20(41):e2308639. doi: 10.1002/smll.202308639. Epub 2023 Dec 21. Small. 2024. PMID: 38126905 Review.
-
Specific cannabinoids revive adaptive immunity by reversing immune evasion mechanisms in metastatic tumours.Front Immunol. 2023 Feb 22;13:982082. doi: 10.3389/fimmu.2022.982082. eCollection 2022. Front Immunol. 2023. PMID: 36923728 Free PMC article.
References
-
- Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer J. 2009;15(3):169–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

