The AMPK signalling pathway coordinates cell growth, autophagy and metabolism
- PMID: 21892142
- PMCID: PMC3249400
- DOI: 10.1038/ncb2329
The AMPK signalling pathway coordinates cell growth, autophagy and metabolism
Abstract
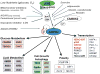
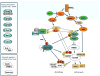
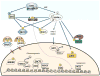
One of the central regulators of cellular and organismal metabolism in eukaryotes is AMP-activated protein kinase (AMPK), which is activated when intracellular ATP production decreases. AMPK has critical roles in regulating growth and reprogramming metabolism, and has recently been connected to cellular processes such as autophagy and cell polarity. Here we review a number of recent breakthroughs in the mechanistic understanding of AMPK function, focusing on a number of newly identified downstream effectors of AMPK.
Figures

Similar articles
-
LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review.
-
5'-AMP-Activated Protein Kinase Signaling in Caenorhabditis elegans.Exp Suppl. 2016;107:375-388. doi: 10.1007/978-3-319-43589-3_15. Exp Suppl. 2016. PMID: 27812988 Review.
-
AMP-activated protein kinase and cancer.Acta Physiol (Oxf). 2009 May;196(1):55-63. doi: 10.1111/j.1748-1716.2009.01980.x. Epub 2009 Feb 25. Acta Physiol (Oxf). 2009. PMID: 19243571 Review.
-
Intracellular signaling of the AMP-activated protein kinase.Adv Protein Chem Struct Biol. 2019;116:171-207. doi: 10.1016/bs.apcsb.2018.12.001. Epub 2019 Jan 14. Adv Protein Chem Struct Biol. 2019. PMID: 31036291 Review.
-
AMPK Regulation of Cell Growth, Apoptosis, Autophagy, and Bioenergetics.Exp Suppl. 2016;107:45-71. doi: 10.1007/978-3-319-43589-3_3. Exp Suppl. 2016. PMID: 27812976 Review.
Cited by
-
The macrophage senescence hypothesis: the role of poor heat shock response in pulmonary inflammation and endothelial dysfunction following chronic exposure to air pollution.Inflamm Res. 2022 Dec;71(12):1433-1448. doi: 10.1007/s00011-022-01647-2. Epub 2022 Oct 20. Inflamm Res. 2022. PMID: 36264363 Review.
-
Bmf upregulation through the AMP-activated protein kinase pathway may protect the brain from seizure-induced cell death.Cell Death Dis. 2013 Apr 25;4(4):e606. doi: 10.1038/cddis.2013.136. Cell Death Dis. 2013. PMID: 23618904 Free PMC article.
-
AMP-Activated Kinase Regulates Lipid Droplet Localization and Stability of Adipose Triglyceride Lipase in C. elegans Dauer Larvae.PLoS One. 2015 Jun 22;10(6):e0130480. doi: 10.1371/journal.pone.0130480. eCollection 2015. PLoS One. 2015. PMID: 26098762 Free PMC article.
-
Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function.Nat Metab. 2020 Sep;2(9):840-848. doi: 10.1038/s42255-020-0188-7. Epub 2020 Mar 30. Nat Metab. 2020. PMID: 32694821 Review.
-
Fyn phosphorylates AMPK to inhibit AMPK activity and AMP-dependent activation of autophagy.Oncotarget. 2016 Nov 15;7(46):74612-74629. doi: 10.18632/oncotarget.11916. Oncotarget. 2016. PMID: 27626315 Free PMC article.
References
-
- Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–942. - PubMed
-
- Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

