Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases
- PMID: 21863020
- PMCID: PMC3173800
- DOI: 10.1038/emboj.2011.307
Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases
Abstract
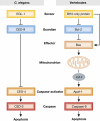
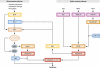
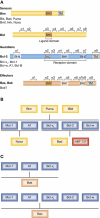
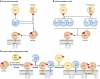
Apoptosis, the major form of programmed cell death in metazoan organisms, plays critical roles in normal development, tissue homeostasis and immunity, and its disturbed regulation contributes to many pathological states, including cancer, autoimmunity, infection and degenerative disorders. In vertebrates, it can be triggered either by engagement of 'death receptors' of the tumour necrosis factor receptor family on the cell surface or by diverse intracellular signals that act upon the Bcl-2 protein family, which controls the integrity of the mitochondrial outer membrane through the complex interactions of family members. Both pathways lead to cellular demolition by dedicated proteases termed caspases. This review discusses the groundbreaking experiments from many laboratories that have clarified cell death regulation and galvanised efforts to translate this knowledge into novel therapeutic strategies for the treatment of malignant and perhaps certain autoimmune and infectious diseases.
Conflict of interest statement
The authors declare that their research at the Walter and Eliza Hall Institute includes a joint programme with Genentech Inc. and Abbott Labs to develop novel anti-cancer therapeutics.
Figures
Similar articles
-
Apoptosis: programmed cell death at a molecular level.Semin Arthritis Rheum. 2003 Jun;32(6):345-69. doi: 10.1053/sarh.2003.50005. Semin Arthritis Rheum. 2003. PMID: 12833244 Review.
-
Does "death receptor" signaling play a role in tumorigenesis and cancer therapy?Oncol Res. 1998;10(11-12):541-50. Oncol Res. 1998. PMID: 10367935 Review.
-
Physician Education: Apoptosis.Oncologist. 1996;1(6):399-401. Oncologist. 1996. PMID: 10388021
-
Mitochondria and apoptosis.Eur J Biochem. 1998 Feb 15;252(1):1-15. doi: 10.1046/j.1432-1327.1998.2520001.x. Eur J Biochem. 1998. PMID: 9523706 Review.
-
The Bcl-2 protein family and its role in the development of neoplastic disease.Exp Gerontol. 2004 Aug;39(8):1125-35. doi: 10.1016/j.exger.2004.04.011. Exp Gerontol. 2004. PMID: 15288687 Review.
Cited by
-
AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC.Cell Death Differ. 2013 Sep;20(9):1149-60. doi: 10.1038/cdd.2013.37. Epub 2013 May 3. Cell Death Differ. 2013. PMID: 23645208 Free PMC article.
-
Eliminating Legionella by inhibiting BCL-XL to induce macrophage apoptosis.Nat Microbiol. 2016 Feb 24;1:15034. doi: 10.1038/nmicrobiol.2015.34. Nat Microbiol. 2016. PMID: 27572165
-
14-3-3 protects against stress-induced apoptosis.Cell Death Dis. 2012 Jul 12;3(7):e348. doi: 10.1038/cddis.2012.90. Cell Death Dis. 2012. PMID: 22785534 Free PMC article.
-
Curcumin induces apoptosis in gallbladder carcinoma cell line GBC-SD cells.Cancer Cell Int. 2013 Jun 26;13(1):64. doi: 10.1186/1475-2867-13-64. Cancer Cell Int. 2013. PMID: 23802572 Free PMC article.
-
Combining paclitaxel with ABT-263 has a synergistic effect on paclitaxel resistant prostate cancer cells.PLoS One. 2015 Mar 26;10(3):e0120913. doi: 10.1371/journal.pone.0120913. eCollection 2015. PLoS One. 2015. PMID: 25811469 Free PMC article.
References
-
- Allan JM, Travis LB (2005) Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer 5: 943–955 - PubMed
-
- Bardwell PD, Gu J, McCarthy D, Wallace C, Bryant S, Goess C, Mathieu S, Grinnell C, Erickson J, Rosenberg SH, Schwartz AJ, Hugunin M, Tarcsa E, Elmore SW, McRae B, Murtaza A, Wang LC, Ghayur T (2009) The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol 182: 7482–7489 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

