Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice
- PMID: 21721026
- PMCID: PMC3213322
- DOI: 10.1002/hep.24501
Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice
Abstract
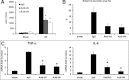
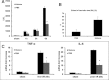
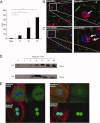
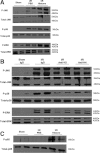
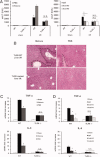
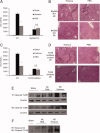
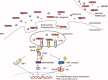
Sterile inflammatory insults are known to activate innate immunity and propagate organ damage through the recognition of extracellular damage-associated molecular pattern (DAMP) molecules. Although DAMPs such as endogenous DNA and nuclear high-mobility group box 1 have been shown to be critical in sterile inflammation, the role of nuclear histone proteins has not yet been investigated. We report that endogenous histones function as DAMPs after ischemic injury through the pattern recognition receptor Toll-like receptor (TLR) 9 to initiate inflammation. Using an in vivo model of hepatic ischemia/reperfusion (I/R) injury, we show that levels of circulating histones are significantly higher after I/R, and that histone neutralization significantly protects against injury. Injection of exogenous histones exacerbates I/R injury through cytotoxic effects mediated by TLR9 and MyD88. In addition, histone administration increases TLR9 activation, whereas neither TLR9 nor MyD88 mutant mice respond to exogenous histones. Furthermore, we demonstrate in vitro that extracellular histones enhance DNA-mediated TLR9 activation in immune cells through a direct interaction.
Conclusion: These novel findings reveal that histones represent a new class of DAMP molecules and serve as a crucial link between initial damage and activation of innate immunity during sterile inflammation.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures

Similar articles
-
Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury.Hepatology. 2010 Feb;51(2):621-32. doi: 10.1002/hep.23365. Hepatology. 2010. PMID: 19902481 Free PMC article.
-
Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury.Hepatology. 2015 Aug;62(2):600-14. doi: 10.1002/hep.27841. Epub 2015 May 29. Hepatology. 2015. PMID: 25855125 Free PMC article.
-
Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection.Hepatology. 2014 May;59(5):1984-1997. doi: 10.1002/hep.26976. Epub 2014 Apr 1. Hepatology. 2014. PMID: 24375466 Free PMC article.
-
The Alarmin Properties of DNA and DNA-associated Nuclear Proteins.Clin Ther. 2016 May;38(5):1029-41. doi: 10.1016/j.clinthera.2016.02.029. Epub 2016 Mar 25. Clin Ther. 2016. PMID: 27021604 Review.
-
Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2014 Apr 15;306(8):F801-11. doi: 10.1152/ajprenal.00469.2013. Epub 2014 Feb 12. Am J Physiol Renal Physiol. 2014. PMID: 24523386 Free PMC article. Review.
Cited by
-
Glutamic-Alanine Rich Glycoprotein from Undaria pinnatifida: A Promising Natural Anti-Inflammatory Agent.Mar Drugs. 2024 Aug 26;22(9):383. doi: 10.3390/md22090383. Mar Drugs. 2024. PMID: 39330264 Free PMC article.
-
Programmed cell death, from liver Ischemia-Reperfusion injury perspective: An overview.Heliyon. 2024 Jun 17;10(13):e32480. doi: 10.1016/j.heliyon.2024.e32480. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39040334 Free PMC article. Review.
-
Trauma-associated extracellular histones mediate inflammation via a MYD88-IRAK1-ERK signaling axis and induce lytic cell death in human adipocytes.Cell Death Dis. 2024 Apr 23;15(4):285. doi: 10.1038/s41419-024-06676-9. Cell Death Dis. 2024. PMID: 38653969 Free PMC article.
-
Formation of memory assemblies through the DNA-sensing TLR9 pathway.Nature. 2024 Apr;628(8006):145-153. doi: 10.1038/s41586-024-07220-7. Epub 2024 Mar 27. Nature. 2024. PMID: 38538785 Free PMC article.
-
M6229 Protects against Extracellular-Histone-Induced Liver Injury, Kidney Dysfunction, and Mortality in a Rat Model of Acute Hyperinflammation.Int J Mol Sci. 2024 Jan 23;25(3):1376. doi: 10.3390/ijms25031376. Int J Mol Sci. 2024. PMID: 38338654 Free PMC article.
References
-
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. - PubMed
-
- Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. - PubMed
-
- Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, et al. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359–363. - PubMed
-
- Lantos J, Foldi V, Roth E, Weber G, Bogar L, Csontos C. Burn trauma induces early HMGB1 release in patients: its correlation with cytokines. Shock. 2010;33:562–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
