Low levels of SIV infection in sooty mangabey central memory CD⁴⁺ T cells are associated with limited CCR5 expression
- PMID: 21706028
- PMCID: PMC3253129
- DOI: 10.1038/nm.2395
Low levels of SIV infection in sooty mangabey central memory CD⁴⁺ T cells are associated with limited CCR5 expression
Abstract
Naturally simian immunodeficiency virus (SIV)-infected sooty mangabeys do not progress to AIDS despite high-level virus replication. We previously showed that the fraction of CD4(+)CCR5(+) T cells is lower in sooty mangabeys compared to humans and macaques. Here we found that, after in vitro stimulation, sooty mangabey CD4(+) T cells fail to upregulate CCR5 and that this phenomenon is more pronounced in CD4(+) central memory T cells (T(CM) cells). CD4(+) T cell activation was similarly uncoupled from CCR5 expression in sooty mangabeys in vivo during acute SIV infection and the homeostatic proliferation that follows antibody-mediated CD4(+) T cell depletion. Sooty mangabey CD4(+) T(CM) cells that express low amounts of CCR5 showed reduced susceptibility to SIV infection both in vivo and in vitro when compared to CD4(+) T(CM) cells of rhesus macaques. These data suggest that low CCR5 expression on sooty mangabey CD4(+) T cells favors the preservation of CD4(+) T cell homeostasis and promotes an AIDS-free status by protecting CD4(+) T(CM) cells from direct virus infection.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

 ). Statistical analyses were performed to compare, in SMs versus RMs, the fraction of CCR5+ cells within each CD4+ and CD8+ T-cell subset.
). Statistical analyses were performed to compare, in SMs versus RMs, the fraction of CCR5+ cells within each CD4+ and CD8+ T-cell subset.
 ) after in vitro stimulations. Fraction of CD4+Ki-67+ (a) and CD4+CCR5+ (b) T-cells following stimulation with ConA/IL-2. Asterisks indicate time points when the fraction of CD4+CCR5+ T-cells was significantly lower in SMs than RMs (p values are detailed in the Result section). (c) Representative dot plots showing the fraction of CD4+CCR5+ T-cells post-stimulation in RMs (top) and SMs (bottom). (d) Flow cytometry dot plots showing Ki-67/CCR5 double staining in a representative RM and SM at 120 hours post stimulation with ConA/IL-2. (e) PBMCs isolated from RMs and SMs were labeled with CFSE prior to mitogen stimulation; levels of CCR5 were analyzed on cells expressing various levels of CFSE dilution at 120 hours post-stimulation. (f) and (g) PBMCs isolated from SMs and RMs were stimulated with recombinant IL-7, and the fraction of CD4+Ki-67+ (f) and CD4+CCR5+ (g) T-cells determined following stimulation. Asterisks indicate time points where, in RMs, the IL-7-induced increase in CD4+CCR5+ T-cells (as compared to baseline) was statistically significant (p values are detailed in the Result section). (h) In a subset of animals, levels of CCR5 mRNA (expressed as CCR5/GAPDH ratio) were determined on purified CD4+ T-cells at 0, 24, 72 and 120 hours post-stimulation with ConA/IL-2. Statistical analyses were performed to compare CCR5/GAPDH ratio between SMs and RMs.
) after in vitro stimulations. Fraction of CD4+Ki-67+ (a) and CD4+CCR5+ (b) T-cells following stimulation with ConA/IL-2. Asterisks indicate time points when the fraction of CD4+CCR5+ T-cells was significantly lower in SMs than RMs (p values are detailed in the Result section). (c) Representative dot plots showing the fraction of CD4+CCR5+ T-cells post-stimulation in RMs (top) and SMs (bottom). (d) Flow cytometry dot plots showing Ki-67/CCR5 double staining in a representative RM and SM at 120 hours post stimulation with ConA/IL-2. (e) PBMCs isolated from RMs and SMs were labeled with CFSE prior to mitogen stimulation; levels of CCR5 were analyzed on cells expressing various levels of CFSE dilution at 120 hours post-stimulation. (f) and (g) PBMCs isolated from SMs and RMs were stimulated with recombinant IL-7, and the fraction of CD4+Ki-67+ (f) and CD4+CCR5+ (g) T-cells determined following stimulation. Asterisks indicate time points where, in RMs, the IL-7-induced increase in CD4+CCR5+ T-cells (as compared to baseline) was statistically significant (p values are detailed in the Result section). (h) In a subset of animals, levels of CCR5 mRNA (expressed as CCR5/GAPDH ratio) were determined on purified CD4+ T-cells at 0, 24, 72 and 120 hours post-stimulation with ConA/IL-2. Statistical analyses were performed to compare CCR5/GAPDH ratio between SMs and RMs.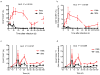
 ) in two in vivo experimental conditions associated with activation of the CD4+ T-cell compartment, i.e., acute SIV-infection and Ab-mediated CD4+ T-cell depletion. Fraction (a) and fold change vs pre-infection, i.e. day 0 (b) of CD4+CCR5+ T-cells at different time points during (i) pathogenic SIVmac239 infection of five RMs and (ii) non-progressive experimental SIVsmm infection of four SMs. SIV-induced CD4+ T-cell activation is associated with an increased fraction of CD4+CCR5+ T-cells in RMs, but not in SMs, with a statistically significant difference in the area under the curve (AUC). Fraction (c) and fold change vs pre-depletion, i.e. day -14 (d) of CD4+CCR5+ T-cells at different time points following Ab-mediated CD4+ T-cell depletion in three uninfected RMs and three uninfected SMs. The AUC of the fraction of CD4+CCR5+ T-cells is significantly higher in RMs than SMs. The dotted lines in (c,d) indicate the 10-day period in which the anti-CD4 antibody was administered.
) in two in vivo experimental conditions associated with activation of the CD4+ T-cell compartment, i.e., acute SIV-infection and Ab-mediated CD4+ T-cell depletion. Fraction (a) and fold change vs pre-infection, i.e. day 0 (b) of CD4+CCR5+ T-cells at different time points during (i) pathogenic SIVmac239 infection of five RMs and (ii) non-progressive experimental SIVsmm infection of four SMs. SIV-induced CD4+ T-cell activation is associated with an increased fraction of CD4+CCR5+ T-cells in RMs, but not in SMs, with a statistically significant difference in the area under the curve (AUC). Fraction (c) and fold change vs pre-depletion, i.e. day -14 (d) of CD4+CCR5+ T-cells at different time points following Ab-mediated CD4+ T-cell depletion in three uninfected RMs and three uninfected SMs. The AUC of the fraction of CD4+CCR5+ T-cells is significantly higher in RMs than SMs. The dotted lines in (c,d) indicate the 10-day period in which the anti-CD4 antibody was administered.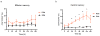
 ) TCM (CD95+CD62L+) and TEM (CD95+CD62L−) cells that were sorted and stimulated with ConA/IL-2. The graphs show the fraction of CD4+CCR5+ TEM (a) and TCM (b) cells at different time points following stimulation. Asterisks indicate time points where values are significantly higher in RMs as compared to SMs.
) TCM (CD95+CD62L+) and TEM (CD95+CD62L−) cells that were sorted and stimulated with ConA/IL-2. The graphs show the fraction of CD4+CCR5+ TEM (a) and TCM (b) cells at different time points following stimulation. Asterisks indicate time points where values are significantly higher in RMs as compared to SMs.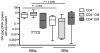

Similar articles
-
Reduced Simian Immunodeficiency Virus Replication in Macrophages of Sooty Mangabeys Is Associated with Increased Expression of Host Restriction Factors.J Virol. 2015 Oct;89(20):10136-44. doi: 10.1128/JVI.00710-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202248 Free PMC article.
-
Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques.J Virol. 1998 Dec;72(12):9597-611. doi: 10.1128/JVI.72.12.9597-9611.1998. J Virol. 1998. PMID: 9811693 Free PMC article.
-
Central memory CD4 T cells are the predominant cell subset resistant to anergy in SIV disease resistant sooty mangabeys.AIDS. 2006 Jan 9;20(2):181-8. doi: 10.1097/01.aids.0000198092.77948.8a. AIDS. 2006. PMID: 16511410
-
SIV Coreceptor Specificity in Natural and Non-Natural Host Infection: Implications for Cell Targeting and Differential Outcomes from Infection.Curr HIV Res. 2018;16(1):41-51. doi: 10.2174/1570162X15666171124121805. Curr HIV Res. 2018. PMID: 29173179 Review.
-
Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS?J Med Primatol. 2005 Oct;34(5-6):243-52. doi: 10.1111/j.1600-0684.2005.00122.x. J Med Primatol. 2005. PMID: 16128919 Review.
Cited by
-
High CCR5 density on central memory CD4+ T cells in acute HIV-1 infection is mostly associated with rapid disease progression.PLoS One. 2012;7(11):e49526. doi: 10.1371/journal.pone.0049526. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185351 Free PMC article.
-
Systems biology of natural simian immunodeficiency virus infections.Curr Opin HIV AIDS. 2012 Jan;7(1):71-8. doi: 10.1097/COH.0b013e32834dde01. Curr Opin HIV AIDS. 2012. PMID: 22134342 Free PMC article. Review.
-
SIVagm infection in wild African green monkeys from South Africa: epidemiology, natural history, and evolutionary considerations.PLoS Pathog. 2013 Jan;9(1):e1003011. doi: 10.1371/journal.ppat.1003011. Epub 2013 Jan 17. PLoS Pathog. 2013. PMID: 23349627 Free PMC article.
-
Slow progression of pediatric HIV associates with early CD8+ T cell PD-1 expression and a stem-like phenotype.JCI Insight. 2023 Feb 8;8(3):e156049. doi: 10.1172/jci.insight.156049. JCI Insight. 2023. PMID: 36602861 Free PMC article.
-
Role of HLA Adaptation in HIV Evolution.Front Immunol. 2016 Jan 18;6:665. doi: 10.3389/fimmu.2015.00665. eCollection 2015. Front Immunol. 2016. PMID: 26834742 Free PMC article. Review.
References
-
- Paiardini MPI, Apetrei C, Silvestri G. Lessons learned from the natural hosts of HIV-related viruses. Annu Rev Med. 2009;60:485–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01-AI76074/AI/NIAID NIH HHS/United States
- T32 AI007632/AI/NIAID NIH HHS/United States
- P01 AI076174/AI/NIAID NIH HHS/United States
- R01 AI066998-06/AI/NIAID NIH HHS/United States
- R01-AI66998/AI/NIAID NIH HHS/United States
- R56-AI087186/AI/NIAID NIH HHS/United States
- P51-RR-00165/RR/NCRR NIH HHS/United States
- R56 AI087186/AI/NIAID NIH HHS/United States
- R01 AI084836/AI/NIAID NIH HHS/United States
- R01 AI066998/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- R01 AI066998-07/AI/NIAID NIH HHS/United States
- P01 AI076174-03/AI/NIAID NIH HHS/United States
- R37 AI066998/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

