Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration of Clostridium perfringens enterotoxin
- PMID: 21692061
- PMCID: PMC3701957
- DOI: 10.1002/cncr.26215
Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration of Clostridium perfringens enterotoxin
Abstract
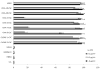
Background: Emerging evidence has suggested that the capability to sustain tumor formation, growth, and chemotherapy resistance in ovarian as well as other human malignancies exclusively resides in a small proportion of tumor cells termed cancer stem cells. During the characterization of CD44(+) ovarian cancer stem cells, we found a high expression of the genes encoding for claudin-4. Because this tight junction protein is the natural high-affinity receptor for Clostridium perfringens enterotoxin (CPE), we have extensively investigated the sensitivity of ovarian cancer stem cells to CPE treatment in vitro and in vivo.
Methods: Real-time polymerase chain reaction and flow cytometry were used to evaluate claudin-3/-4 expression in ovarian cancer stem cells. Small interfering RNA knockdown experiments and MTS assays were used to evaluate CPE-induced cytotoxicity against ovarian cancer stem cell lines in vitro. C.B-17/SCID mice harboring ovarian cancer stem cell xenografts were used to evaluate CPE therapeutic activity in vivo.
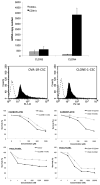
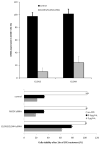
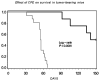
Results: CD44(+) ovarian cancer stem cells expressed claudin-4 gene at significantly higher levels than matched autologous CD44(-) ovarian cancer cells, and regardless of their higher resistance to chemotherapeutic agents died within 1 hour after exposure to 1.0 μg/mL of CPE in vitro. Conversely, small-interfering RNA-mediated knockdown of claudin-3/-4 expression in CD44(+) cancer stem cells significantly protected cancer stem cells from CPE-induced cytotoxicity. Importantly, multiple intraperitoneal administrations of sublethal doses of CPE in mice harboring xenografts of chemotherapy-resistant CD44(+) ovarian cancer stem cells had a significant inhibitory effect on tumor progression leading to the cure and/or long-term survival of all treated animals (ie, 100% reduction in tumor burden in 50% of treated mice; P < .0001).
Conclusions: CPE may represent an unconventional, potentially highly effective strategy to eradicate chemotherapy-resistant cancer stem cells.
Copyright © 2011 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
Figures
Similar articles
-
Treatment of chemotherapy-resistant human ovarian cancer xenografts in C.B-17/SCID mice by intraperitoneal administration of Clostridium perfringens enterotoxin.Cancer Res. 2005 May 15;65(10):4334-42. doi: 10.1158/0008-5472.CAN-04-3472. Cancer Res. 2005. PMID: 15899825
-
Clostridium perfringens enterotoxin carboxy-terminal fragment is a novel tumor-homing peptide for human ovarian cancer.BMC Cancer. 2010 Jul 2;10:349. doi: 10.1186/1471-2407-10-349. BMC Cancer. 2010. PMID: 20598131 Free PMC article.
-
Cytotoxicity of Clostridium perfringens enterotoxin depends on the conditions of claudin-4 in ovarian carcinoma cells.Exp Cell Res. 2018 Oct 1;371(1):278-286. doi: 10.1016/j.yexcr.2018.08.024. Epub 2018 Aug 22. Exp Cell Res. 2018. PMID: 30142326
-
Claudins overexpression in ovarian cancer: potential targets for Clostridium Perfringens Enterotoxin (CPE) based diagnosis and therapy.Int J Mol Sci. 2013 May 17;14(5):10412-37. doi: 10.3390/ijms140510412. Int J Mol Sci. 2013. PMID: 23685873 Free PMC article. Review.
-
On the interaction of Clostridium perfringens enterotoxin with claudins.Toxins (Basel). 2010 Jun;2(6):1336-56. doi: 10.3390/toxins2061336. Epub 2010 Jun 8. Toxins (Basel). 2010. PMID: 22069641 Free PMC article. Review.
Cited by
-
Ovarian Tumor Cell Expression of Claudin-4 Reduces Apoptotic Response to Paclitaxel.Mol Cancer Res. 2019 Mar;17(3):741-750. doi: 10.1158/1541-7786.MCR-18-0451. Epub 2019 Jan 3. Mol Cancer Res. 2019. PMID: 30606772 Free PMC article.
-
Loss of Claudin-4 Reduces DNA Damage Repair and Increases Sensitivity to PARP Inhibitors.Mol Cancer Ther. 2022 Apr 1;21(4):647-657. doi: 10.1158/1535-7163.MCT-21-0827. Mol Cancer Ther. 2022. PMID: 35373300 Free PMC article.
-
Emerging roles of claudins in human cancer.Int J Mol Sci. 2013 Sep 4;14(9):18148-80. doi: 10.3390/ijms140918148. Int J Mol Sci. 2013. PMID: 24009024 Free PMC article. Review.
-
Nanomaterial-assisted oncolytic bacteria in solid tumor diagnosis and therapeutics.Bioeng Transl Med. 2024 Apr 17;9(4):e10672. doi: 10.1002/btm2.10672. eCollection 2024 Jul. Bioeng Transl Med. 2024. PMID: 39036084 Free PMC article. Review.
-
Serglycin in human cancers.Chin J Cancer. 2011 Sep;30(9):585-9. doi: 10.5732/cjc.011.10314. Chin J Cancer. 2011. PMID: 21880179 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Copeland L. Epithelial ovarian cancer. In: DiSaia PJ, Creasman WT, editors. Clinical Gynecologic Oncology. St Louis: Mosby Year Book; 1997. pp. 313–367.
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. - PubMed
-
- Hermann PC, Huber SL, Heeschen C. Metastatic cancer stem cells: a new target for anti-cancer therapy? Cell Cycle. 2008;7:188–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

