Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats
- PMID: 21686174
- PMCID: PMC3116180
- DOI: 10.3390/ijms12053133
Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats
Abstract
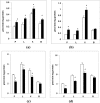
We have previously shown a tissue-specific increase in oxidative stress in the early stages of streptozotocin (STZ)-induced diabetic rats. In this study, we investigated oxidative stress-related long-term complications and mitochondrial dysfunctions in the different tissues of STZ-induced diabetic rats (>15 mM blood glucose for 8 weeks). These animals showed a persistent increase in reactive oxygen and nitrogen species (ROS and RNS, respectively) production. Oxidative protein carbonylation was also increased with the maximum effect observed in the pancreas of diabetic rats. The activities of mitochondrial respiratory enzymes ubiquinol: cytochrome c oxidoreductase (Complex III) and cytochrome c oxidase (Complex IV) were significantly decreased while that of NADH:ubiquinone oxidoreductase (Complex I) and succinate:ubiquinone oxidoreductase (Complex II) were moderately increased in diabetic rats, which was confirmed by the increased expression of the 70 kDa Complex II sub-unit. Mitochondrial matrix aconitase, a ROS sensitive enzyme, was markedly inhibited in the diabetic rat tissues. Increased expression of oxidative stress marker proteins Hsp-70 and HO-1 was also observed along with increased expression of nitric oxide synthase. These results suggest that mitochondrial respiratory complexes may play a critical role in ROS/RNS homeostasis and oxidative stress related changes in type 1 diabetes and may have implications in the etiology of diabetes and its complications.
Keywords: NO; ROS; diabetes; mitochondrial respiration; oxidative stress.
Figures
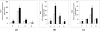
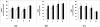
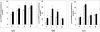

Similar articles
-
Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats.Diabetes. 2011 Jan;60(1):288-97. doi: 10.2337/db10-0818. Epub 2010 Sep 28. Diabetes. 2011. PMID: 20876714 Free PMC article.
-
Protective effect of boldine on oxidative mitochondrial damage in streptozotocin-induced diabetic rats.Pharmacol Res. 2000 Oct;42(4):361-71. doi: 10.1006/phrs.2000.0705. Pharmacol Res. 2000. PMID: 10987997
-
Mitochondrial dysfunction in brain cortex mitochondria of STZ-diabetic rats: effect of l-Arginine.Neurochem Res. 2013 Dec;38(12):2570-80. doi: 10.1007/s11064-013-1172-3. Epub 2013 Nov 5. Neurochem Res. 2013. PMID: 24190597
-
Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment.Diabetes. 2010 Apr;59(4):1082-91. doi: 10.2337/db09-1299. Epub 2010 Jan 26. Diabetes. 2010. PMID: 20103706 Free PMC article.
-
Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase.Can J Physiol Pharmacol. 2010 Mar;88(3):241-8. doi: 10.1139/Y10-018. Can J Physiol Pharmacol. 2010. PMID: 20393589 Review.
Cited by
-
Quality Matters? The Involvement of Mitochondrial Quality Control in Cardiovascular Disease.Front Cell Dev Biol. 2021 Mar 22;9:636295. doi: 10.3389/fcell.2021.636295. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33829016 Free PMC article. Review.
-
Overlapped metabolic and therapeutic links between Alzheimer and diabetes.Mol Neurobiol. 2013 Feb;47(1):399-424. doi: 10.1007/s12035-012-8352-z. Epub 2012 Sep 26. Mol Neurobiol. 2013. PMID: 23011810 Review.
-
Exercise alleviates diabetic complications by inhibiting oxidative stress-mediated signaling cascade and mitochondrial metabolic stress in GK diabetic rat tissues.Front Physiol. 2022 Dec 1;13:1052608. doi: 10.3389/fphys.2022.1052608. eCollection 2022. Front Physiol. 2022. PMID: 36531176 Free PMC article.
-
The Use of Natural Compounds as a Strategy to Counteract Oxidative Stress in Animal Models of Diabetes Mellitus.Int J Mol Sci. 2021 Jun 29;22(13):7009. doi: 10.3390/ijms22137009. Int J Mol Sci. 2021. PMID: 34209800 Free PMC article. Review.
-
Jabuticaba [Plinia trunciflora (O. Berg) Kausel] Protects Liver of Diabetic Rats Against Mitochondrial Dysfunction and Oxidative Stress Through the Modulation of SIRT3 Expression.Front Physiol. 2021 Jul 6;12:665747. doi: 10.3389/fphys.2021.665747. eCollection 2021. Front Physiol. 2021. PMID: 34295258 Free PMC article.
References
-
- Baynes J. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. - PubMed
-
- Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Rad. Biol. Med. 2006;41:177–184. - PubMed
-
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharm. 2006;212:167–178. - PubMed
-
- Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin. Chem. Lab. Med. 2003;41:1144–1149. - PubMed
-
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

