Reactive oxygen species hydrogen peroxide mediates Kaposi's sarcoma-associated herpesvirus reactivation from latency
- PMID: 21625536
- PMCID: PMC3098240
- DOI: 10.1371/journal.ppat.1002054
Reactive oxygen species hydrogen peroxide mediates Kaposi's sarcoma-associated herpesvirus reactivation from latency
Abstract
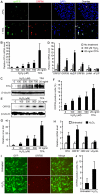
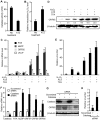
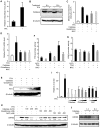
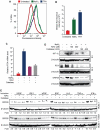
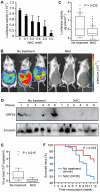
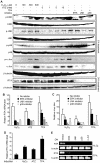
Kaposi's sarcoma-associated herpesvirus (KSHV) establishes a latent infection in the host following an acute infection. Reactivation from latency contributes to the development of KSHV-induced malignancies, which include Kaposi's sarcoma (KS), the most common cancer in untreated AIDS patients, primary effusion lymphoma and multicentric Castleman's disease. However, the physiological cues that trigger KSHV reactivation remain unclear. Here, we show that the reactive oxygen species (ROS) hydrogen peroxide (H₂O₂) induces KSHV reactivation from latency through both autocrine and paracrine signaling. Furthermore, KSHV spontaneous lytic replication, and KSHV reactivation from latency induced by oxidative stress, hypoxia, and proinflammatory and proangiogenic cytokines are mediated by H₂O₂. Mechanistically, H₂O₂ induction of KSHV reactivation depends on the activation of mitogen-activated protein kinase ERK1/2, JNK, and p38 pathways. Significantly, H₂O₂ scavengers N-acetyl-L-cysteine (NAC), catalase and glutathione inhibit KSHV lytic replication in culture. In a mouse model of KSHV-induced lymphoma, NAC effectively inhibits KSHV lytic replication and significantly prolongs the lifespan of the mice. These results directly relate KSHV reactivation to oxidative stress and inflammation, which are physiological hallmarks of KS patients. The discovery of this novel mechanism of KSHV reactivation indicates that antioxidants and anti-inflammation drugs could be promising preventive and therapeutic agents for effectively targeting KSHV replication and KSHV-related malignancies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
FoxO1 Suppresses Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication and Controls Viral Latency.J Virol. 2019 Jan 17;93(3):e01681-18. doi: 10.1128/JVI.01681-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30404794 Free PMC article.
-
Reactivation of Kaposi's sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways.Virology. 2008 Feb 5;371(1):139-54. doi: 10.1016/j.virol.2007.09.040. Epub 2007 Oct 26. Virology. 2008. PMID: 17964626 Free PMC article.
-
Oxidant species are involved in T/B-mediated ERK1/2 phosphorylation that activates p53-p21 axis to promote KSHV lytic cycle in PEL cells.Free Radic Biol Med. 2017 Nov;112:327-335. doi: 10.1016/j.freeradbiomed.2017.08.005. Epub 2017 Aug 8. Free Radic Biol Med. 2017. PMID: 28801242
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
-
[Replication Machinery of Kaposi's Sarcoma-associated Herpesvirus and Drug Discovery Research].Yakugaku Zasshi. 2019;139(1):69-73. doi: 10.1248/yakushi.18-00164-2. Yakugaku Zasshi. 2019. PMID: 30606932 Review. Japanese.
Cited by
-
Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses.PLoS Pathog. 2015 Jul 28;11(7):e1005064. doi: 10.1371/journal.ppat.1005064. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26218605 Free PMC article.
-
Fine-Tuning of the Kaposi's Sarcoma-Associated Herpesvirus Life Cycle in Neighboring Cells through the RTA-JAG1-Notch Pathway.PLoS Pathog. 2016 Oct 19;12(10):e1005900. doi: 10.1371/journal.ppat.1005900. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27760204 Free PMC article.
-
Targeting Kaposi's Sarcoma-Associated Herpesvirus ORF21 Tyrosine Kinase and Viral Lytic Reactivation by Tyrosine Kinase Inhibitors Approved for Clinical Use.J Virol. 2020 Feb 14;94(5):e01791-19. doi: 10.1128/JVI.01791-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31826996 Free PMC article.
-
How Oncogenic Viruses Exploit p62-Mediated Selective Autophagy for Cancer Development.Ann Immunol Immunother. 2021;3(1):134. Epub 2021 Mar 5. Ann Immunol Immunother. 2021. PMID: 34632457 Free PMC article. No abstract available.
-
The regulation of KSHV lytic reactivation by viral and cellular factors.Curr Opin Virol. 2022 Feb;52:39-47. doi: 10.1016/j.coviro.2021.11.004. Epub 2021 Dec 3. Curr Opin Virol. 2022. PMID: 34872030 Free PMC article. Review.
References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

