The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells
- PMID: 21625531
- PMCID: PMC3098827
- DOI: 10.1371/journal.pone.0019823
The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells
Abstract
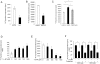
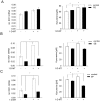
1-methyl-D-tryptophan (1-D-MT) is currently being used in clinical trials in patients with relapsed or refractory solid tumors with the aim of inhibiting indoleamine-2,3-dioxygenase (IDO)-mediated tumor immune escape. IDO is expressed in tumors and tumor-draining lymph nodes and degrades tryptophan (trp) to create an immunsuppressive micromilieu both by depleting trp and by accumulating immunosuppressive metabolites of the kynurenine (kyn) pathway. Here we show that proliferation of alloreactive T-cells cocultured with IDO1-positive human cancer cells paradoxically was inhibited by 1-D-MT. Surprisingly incubation with 1-D-MT increased kyn production of human cancer cells. Cell-free assays revealed that 1-D-MT did not alter IDO1 enzymatic activity. Instead, 1-D-MT induced IDO1 mRNA and protein expression through pathways involving p38 MAPK and JNK signalling. Treatment of cancer patients with 1-D-MT has transcriptional effects that may promote rather than suppress anti-tumor immune escape by increasing IDO1 in the cancer cells. These off-target effects should be carefully analyzed in the ongoing clinical trials with 1-D-MT.
Conflict of interest statement
Figures





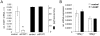
Similar articles
-
IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism.Cancer Immunol Immunother. 2009 Jan;58(1):153-7. doi: 10.1007/s00262-008-0513-6. Epub 2008 Apr 17. Cancer Immunol Immunother. 2009. PMID: 18418598 Free PMC article.
-
1-MT inhibits the invasion of CBP-resistant ovarian cancer cells via down-regulating IDO expression and re-activating immune cells function.BMC Pharmacol Toxicol. 2020 Sep 11;21(1):67. doi: 10.1186/s40360-020-00439-w. BMC Pharmacol Toxicol. 2020. PMID: 32912307 Free PMC article.
-
The expanding roles of 1-methyl-tryptophan (1-MT): in addition to inhibiting kynurenine production, 1-MT activates the synthesis of melatonin in skin cells.FEBS J. 2013 Oct;280(19):4782-92. doi: 10.1111/febs.12444. Epub 2013 Aug 22. FEBS J. 2013. PMID: 23879623
-
Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer.Int Rev Cell Mol Biol. 2018;336:175-203. doi: 10.1016/bs.ircmb.2017.07.004. Epub 2017 Sep 21. Int Rev Cell Mol Biol. 2018. PMID: 29413890 Free PMC article. Review.
-
Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors and PROTAC-based degraders for cancer therapy.Eur J Med Chem. 2022 Jan 5;227:113967. doi: 10.1016/j.ejmech.2021.113967. Epub 2021 Nov 2. Eur J Med Chem. 2022. PMID: 34752953 Review.
Cited by
-
Kynurenines as a Novel Target for the Treatment of Inflammatory Disorders.Cells. 2024 Jul 26;13(15):1259. doi: 10.3390/cells13151259. Cells. 2024. PMID: 39120289 Free PMC article. Review.
-
IDO1 in cancer: a Gemini of immune checkpoints.Cell Mol Immunol. 2018 May;15(5):447-457. doi: 10.1038/cmi.2017.143. Epub 2018 Jan 29. Cell Mol Immunol. 2018. PMID: 29375124 Free PMC article. Review.
-
No patient left behind: The promise of immune priming with epigenetic agents.Oncoimmunology. 2017 Aug 30;6(10):e1315486. doi: 10.1080/2162402X.2017.1315486. eCollection 2017. Oncoimmunology. 2017. PMID: 29123948 Free PMC article. Review.
-
A simple LC-MS/MS method for determination of kynurenine and tryptophan concentrations in human plasma from HIV-infected patients.Bioanalysis. 2013 Jun;5(11):1397-407. doi: 10.4155/bio.13.74. Bioanalysis. 2013. PMID: 23742309 Free PMC article.
-
Trial watch: IDO inhibitors in cancer therapy.Oncoimmunology. 2014 Dec 15;3(10):e957994. doi: 10.4161/21624011.2014.957994. eCollection 2014 Nov. Oncoimmunology. 2014. PMID: 25941578 Free PMC article. Review.
References
-
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. - PubMed
-
- Platten M, Ho PP, Youssef S, Fontoura P, Garren H, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. - PubMed
-
- Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect. 2009;11:133–141. - PubMed
-
- Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

