DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin
- PMID: 21511815
- PMCID: PMC3159438
- DOI: 10.1093/nar/gkr230
DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin
Abstract
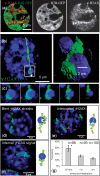
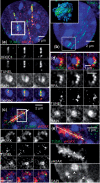
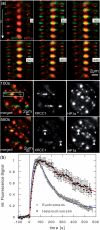
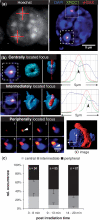
DNA double-strand breaks (DSBs) can induce chromosomal aberrations and carcinogenesis and their correct repair is crucial for genetic stability. The cellular response to DSBs depends on damage signaling including the phosphorylation of the histone H2AX (γH2AX). However, a lack of γH2AX formation in heterochromatin (HC) is generally observed after DNA damage induction. Here, we examine γH2AX and repair protein foci along linear ion tracks traversing heterochromatic regions in human or murine cells and find the DSBs and damage signal streaks bending around highly compacted DNA. Given the linear particle path, such bending indicates a relocation of damage from the initial induction site to the periphery of HC. Real-time imaging of the repair protein GFP-XRCC1 confirms fast recruitment to heterochromatic lesions inside murine chromocenters. Using single-ion microirradiation to induce localized DSBs directly within chromocenters, we demonstrate that H2AX is early phosphorylated within HC, but the damage site is subsequently expelled from the center to the periphery of chromocenters within ∼ 20 min. While this process can occur in the absence of ATM kinase, the repair of DSBs bordering HC requires the protein. Finally, we describe a local decondensation of HC at the sites of ion hits, potentially allowing for DSB movement via physical forces.
Figures

Similar articles
-
The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review.
-
ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin.Mol Cell. 2008 Jul 25;31(2):167-77. doi: 10.1016/j.molcel.2008.05.017. Mol Cell. 2008. PMID: 18657500
-
The impact of heterochromatin on DSB repair.Biochem Soc Trans. 2009 Jun;37(Pt 3):569-76. doi: 10.1042/BST0370569. Biochem Soc Trans. 2009. PMID: 19442252
-
Analysis of human syndromes with disordered chromatin reveals the impact of heterochromatin on the efficacy of ATM-dependent G2/M checkpoint arrest.Mol Cell Biol. 2011 Oct;31(19):4022-35. doi: 10.1128/MCB.05289-11. Epub 2011 Jul 26. Mol Cell Biol. 2011. PMID: 21791604 Free PMC article.
-
DNA double-strand break repair within heterochromatic regions.Biochem Soc Trans. 2012 Feb;40(1):173-8. doi: 10.1042/BST20110631. Biochem Soc Trans. 2012. PMID: 22260685 Review.
Cited by
-
DNA double-strand break movement in heterochromatin depends on the histone acetyltransferase dGcn5.Nucleic Acids Res. 2024 Oct 28;52(19):11753-11767. doi: 10.1093/nar/gkae775. Nucleic Acids Res. 2024. PMID: 39258543 Free PMC article.
-
Replication fork stalling in late S-phase elicits nascent strand degradation by DNA mismatch repair.Nucleic Acids Res. 2024 Oct 14;52(18):10999-11013. doi: 10.1093/nar/gkae721. Nucleic Acids Res. 2024. PMID: 39180395 Free PMC article.
-
"Off-pore" nucleoporins relocalize heterochromatic breaks through phase separation.bioRxiv [Preprint]. 2024 Jul 18:2023.12.07.570729. doi: 10.1101/2023.12.07.570729. bioRxiv. 2024. PMID: 39071440 Free PMC article. Preprint.
-
Nup153 is not required for anchoring heterochromatic DSBs to the nuclear periphery.MicroPubl Biol. 2024 Apr 26;2024:10.17912/micropub.biology.001176. doi: 10.17912/micropub.biology.001176. eCollection 2024. MicroPubl Biol. 2024. PMID: 38737725 Free PMC article.
-
Dose-Dependent Transcriptional Response to Ionizing Radiation Is Orchestrated with DNA Repair within the Nuclear Space.Int J Mol Sci. 2024 Jan 12;25(2):970. doi: 10.3390/ijms25020970. Int J Mol Sci. 2024. PMID: 38256047 Free PMC article.
References
-
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 2001;276:42462–42467. - PubMed
-
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Löbrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

