Membrane-associated ubiquitin ligase complex containing gp78 mediates sterol-accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase
- PMID: 21343306
- PMCID: PMC3083207
- DOI: 10.1074/jbc.M110.211326
Membrane-associated ubiquitin ligase complex containing gp78 mediates sterol-accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase
Abstract
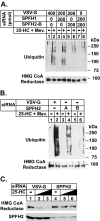
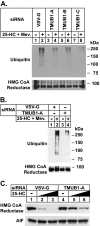
The endoplasmic reticulum (ER)-associated degradation (ERAD) pathway in the yeast Saccharomyces cerevisiae is mediated by two membrane-bound ubiquitin ligases, Doa10 and Hrd1. These enzymes are found in distinct multiprotein complexes that allow them to recognize and target a variety of substrates for proteasomal degradation. Although multiprotein complexes containing mammalian ERAD ubiquitin ligases likely exist, they have yet to be identified and characterized in detail. Here, we identify two ER membrane proteins, SPFH2 and TMUB1, as associated proteins of mammalian gp78, a membrane-bound ubiquitin ligase that bears significant sequence homology with mammalian Hrd1 and mediates sterol-accelerated ERAD of the cholesterol biosynthetic enzyme HMG-CoA reductase. Co-immunoprecipitation studies indicate that TMUB1 bridges SPFH2 to gp78 in ER membranes. The functional significance of these interactions is revealed by the observation that RNA interference (RNAi)-mediated knockdown of SPFH2 and TMUB1 blunts both the sterol-induced ubiquitination and degradation of endogenous reductase in HEK-293 cells. These studies mark the initial steps in the characterization of the mammalian gp78 ubiquitin ligase complex, the further elucidation of which may yield important insights into mechanisms underlying gp78-mediated ERAD.
Figures




Similar articles
-
Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase.Mol Cell. 2005 Sep 16;19(6):829-40. doi: 10.1016/j.molcel.2005.08.009. Mol Cell. 2005. PMID: 16168377
-
Sterol-induced degradation of HMG CoA reductase depends on interplay of two Insigs and two ubiquitin ligases, gp78 and Trc8.Proc Natl Acad Sci U S A. 2011 Dec 20;108(51):20503-8. doi: 10.1073/pnas.1112831108. Epub 2011 Dec 5. Proc Natl Acad Sci U S A. 2011. PMID: 22143767 Free PMC article.
-
Ancient ubiquitous protein-1 mediates sterol-induced ubiquitination of 3-hydroxy-3-methylglutaryl CoA reductase in lipid droplet-associated endoplasmic reticulum membranes.Mol Biol Cell. 2013 Feb;24(3):169-83. doi: 10.1091/mbc.E12-07-0564. Epub 2012 Dec 5. Mol Biol Cell. 2013. PMID: 23223569 Free PMC article.
-
Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase.Crit Rev Biochem Mol Biol. 2010 Jun;45(3):185-98. doi: 10.3109/10409238.2010.485605. Crit Rev Biochem Mol Biol. 2010. PMID: 20482385 Free PMC article. Review.
-
Underlying mechanisms for sterol-induced ubiquitination and ER-associated degradation of HMG CoA reductase.Semin Cell Dev Biol. 2018 Sep;81:121-128. doi: 10.1016/j.semcdb.2017.10.019. Epub 2017 Nov 7. Semin Cell Dev Biol. 2018. PMID: 29107682 Free PMC article. Review.
Cited by
-
Proteomics analysis of lung tissue reveals protein makers for the lung injury of adjuvant arthritis rats.Mol Med Rep. 2023 Sep;28(3):163. doi: 10.3892/mmr.2023.13051. Epub 2023 Jul 14. Mol Med Rep. 2023. PMID: 37449522 Free PMC article.
-
Clustered hydrophobic amino acids in amphipathic helices mediate erlin1/2 complex assembly.Biochem Biophys Res Commun. 2011 Nov 11;415(1):135-40. doi: 10.1016/j.bbrc.2011.10.032. Epub 2011 Oct 12. Biochem Biophys Res Commun. 2011. PMID: 22020079 Free PMC article.
-
Regulation and metabolic engineering strategies for permeases of Saccharomyces cerevisiae.World J Microbiol Biotechnol. 2019 Jul 8;35(7):112. doi: 10.1007/s11274-019-2684-z. World J Microbiol Biotechnol. 2019. PMID: 31286266 Review.
-
A novel autosomal dominant ERLIN2 variant activates endoplasmic reticulum stress in a Chinese HSP family.Ann Clin Transl Neurol. 2023 Nov;10(11):2139-2148. doi: 10.1002/acn3.51902. Epub 2023 Sep 27. Ann Clin Transl Neurol. 2023. PMID: 37752894 Free PMC article.
-
Expanding SPG18 clinical spectrum: autosomal dominant mutation causes complicated hereditary spastic paraplegia in a large family.Neurol Sci. 2024 Sep;45(9):4373-4381. doi: 10.1007/s10072-024-07500-0. Epub 2024 Apr 12. Neurol Sci. 2024. PMID: 38607533 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

