A dense network of dendritic cells populates the murine epididymis
- PMID: 21310816
- PMCID: PMC3657760
- DOI: 10.1530/REP-10-0493
A dense network of dendritic cells populates the murine epididymis
Abstract
One of the most intriguing aspects of male reproductive physiology is the ability to generate spermatogenic cells - which are 'foreign' to the host - without triggering immune activation. After leaving the testis, spermatozoa enter the epididymis where they mature and are stored. In this study, we report a previously unrecognized dense network of dendritic cells (DCs) located at the base of the epididymal epithelium. This network was detected in transgenic mice expressing CD11c-EYFP and CX3CR1-GFP reporters. Epididymal DCs (eDCs) establish intimate interactions with the epithelium and project long dendrites between epithelial cells toward the lumen. We show that isolated eDCs express numerous leukocyte markers described previously in other organs that are in contact with the external environment, and present and cross-present ovalbumin to T cells in vitro. eDCs are, therefore, strategically positioned to regulate the complex interplay between immune tolerance and activation, a balance that is fundamental to male fertility.
Figures
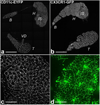
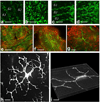
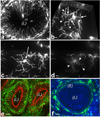

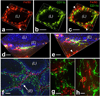
Similar articles
-
Immunoregulatory mechanisms between epithelial clear cells and mononuclear phagocytes in the epididymis.Andrology. 2024 Jul;12(5):949-963. doi: 10.1111/andr.13509. Epub 2023 Aug 12. Andrology. 2024. PMID: 37572347 Review.
-
Mononuclear phagocytes rapidly clear apoptotic epithelial cells in the proximal epididymis.Andrology. 2014 Sep;2(5):755-62. doi: 10.1111/j.2047-2927.2014.00251.x. Epub 2014 Aug 1. Andrology. 2014. PMID: 25082073 Free PMC article.
-
From initial segment to cauda: a regional characterization of mouse epididymal CD11c+ mononuclear phagocytes based on immune phenotype and function.Am J Physiol Cell Physiol. 2020 Dec 1;319(6):C997-C1010. doi: 10.1152/ajpcell.00392.2020. Epub 2020 Sep 29. Am J Physiol Cell Physiol. 2020. PMID: 32991210 Free PMC article.
-
Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis.Biol Reprod. 2014 May 1;90(5):90. doi: 10.1095/biolreprod.113.116681. Print 2014 May. Biol Reprod. 2014. PMID: 24648397 Free PMC article.
-
Macrophages and dendritic cells in the post-testicular environment.Cell Tissue Res. 2016 Jan;363(1):97-104. doi: 10.1007/s00441-015-2270-0. Epub 2015 Sep 4. Cell Tissue Res. 2016. PMID: 26337514 Free PMC article. Review.
Cited by
-
Effects of Heparan sulfate acetyl-CoA: Alpha-glucosaminide N-acetyltransferase (HGSNAT) inactivation on the structure and function of epithelial and immune cells of the testis and epididymis and sperm parameters in adult mice.PLoS One. 2023 Sep 27;18(9):e0292157. doi: 10.1371/journal.pone.0292157. eCollection 2023. PLoS One. 2023. PMID: 37756356 Free PMC article.
-
Regulatory T cells play a crucial role in maintaining sperm tolerance and male fertility.Proc Natl Acad Sci U S A. 2023 Sep 12;120(37):e2306797120. doi: 10.1073/pnas.2306797120. Epub 2023 Sep 7. Proc Natl Acad Sci U S A. 2023. PMID: 37676910 Free PMC article.
-
Immunoregulatory mechanisms between epithelial clear cells and mononuclear phagocytes in the epididymis.Andrology. 2024 Jul;12(5):949-963. doi: 10.1111/andr.13509. Epub 2023 Aug 12. Andrology. 2024. PMID: 37572347 Review.
-
Three-dimensional imaging of vascular development in the mouse epididymis.Elife. 2023 Jun 13;12:e82748. doi: 10.7554/eLife.82748. Elife. 2023. PMID: 37310207 Free PMC article.
-
Myd88 Signaling Is Involved in the Inflammatory Response in LPS-Induced Mouse Epididymitis and Bone-Marrow-Derived Dendritic Cells.Int J Mol Sci. 2023 Apr 25;24(9):7838. doi: 10.3390/ijms24097838. Int J Mol Sci. 2023. PMID: 37175545 Free PMC article.
References
-
- Abe K, Takano H, Ito T. Microvasculature of the mouse epididymis, with special reference to fenestrated capillaries localized in the initial segment. Anat Rec. 1984;209:209–218. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Barratt CL, Bolton AE, Cooke ID. Functional significance of white blood cells in the male and female reproductive tract. Hum Reprod. 1990;5:639–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL095612/HL/NHLBI NIH HHS/United States
- R56 AI084880/AI/NIAID NIH HHS/United States
- R01 DK068181/DK/NIDDK NIH HHS/United States
- P50 CA086355/CA/NCI NIH HHS/United States
- DK43351/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- R01 DK085715/DK/NIDDK NIH HHS/United States
- CA086355/CA/NCI NIH HHS/United States
- R01 AI084880/AI/NIAID NIH HHS/United States
- R01 HD069623/HD/NICHD NIH HHS/United States
- DK38452/DK/NIDDK NIH HHS/United States
- R01HL095612/HL/NHLBI NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- AI084880/AI/NIAID NIH HHS/United States
- DK085715/DK/NIDDK NIH HHS/United States
- P01 DK038452/DK/NIDDK NIH HHS/United States
- DK57521/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

